Abstract
Purpose
Use of mesh is essential in hernia repair. A common complication after hernia repair is surgical site infection (SSI), which poses a risk in spreading to the mesh, possibly causing mesh infection. Topical antimicrobial pretreatment of mesh may potentially reduce SSI risk in hernia repair and has shown promising results in in vitro and in vivo studies. Clinical evidence, however, is more important. This systematic review aims to provide an overview of available clinical evidence for antimicrobial pretreated mesh in hernia repair surgery to reduce SSI.
Methods
We report in accordance with PRISMA guidelines. CENTRAL, EMBASE, CINAHL and PubMed were searched up to October 2023 for studies that investigated the use of antimicrobial pretreated mesh on SSI incidence in adults undergoing hernia repair. The primary outcome was SSI incidence. We also collected data on pathogen involvement, hernia recurrence, and mesh infection. A meta-analysis on SSI risk and GRADE-assessment was performed of eligible studies.
Results
We identified 11 eligible studies (n = 2660 patients); 5 randomized trials and 6 cohort studies. Investigated interventions included pre-coated mesh, antibiotic carriers, mesh soaked or irrigated with antibiotic or antiseptic solution. Meta-analysis showed no significant reduction in SSI for antibiotic pretreated polypropylene mesh (RR 0.76 [95% CI 0.27; 2.09]; I2 50%).
Conclusion
Data on topical mesh pretreatment to reduce SSI risk after hernia repair is limited. Very low certainty evidence from randomized trials in hernia repair surgery shows no significant benefit for antibiotic mesh pretreatment for SSI reduction, but data are imprecise due to optimal information size not being met.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hernia repair surgery impacts over 20 million people worldwide yearly [1]. Surgical site infection (SSI) remains one of the biggest challenges within the hernia repair field, leading to increase in morbidity, mortality and costs [2]. Over the years, different methods and techniques have been developed to improve surgical outcomes and minimize the occurrence of postoperative complications, such as SSI.
The development and use of mesh prosthetics has had great impact on the field of hernia surgery [3]. These mesh, available in a large variety of compositions (i.e., biologic, various synthetic components), can enhance the structural integrity of the hernia repair, providing stability and reinforcement to the abdominal wall. The incorporation of surgical mesh into hernia repair procedures, both in groin and ventral/incisional hernias, has led to reduced hernia recurrence rates [4]. However, concerns regarding the risk of SSI still remain relevant, as the use of prosthetic materials provides an opportunity for bacteria to attach to the surgical mesh and develop biofilms [5]. These infections are often caused by bacteria that are naturally present in the skin flora, including Staphylococcus aureus, Staphylococcus epidermis, Escherichia coli, and Enterococcus species [6].
In response to these concerns, treatment with intravenous antibiotic prophylaxis is predominantly used to mitigate infection rates [7]. Currently, there is a substantial body of research dedicated to systemic antibiotic prophylaxis. Its use has become standard of care in open ventral hernia repairs, while the efficacy for groin hernia surgery remains equivocal, raising concerns about its systemic side effects [8]. Recently, there has been a focus shift toward antimicrobial properties of the surgical mesh itself, exploring the incorporation of various antimicrobial compounds, such as antimicrobial metals (e.g., silver, titanium, zinc and gold), antiseptics (e.g., povidone-iodine or chlorhexidine) and antibiotics (e.g., ampicillin, gentamicin, cefazolin, rifampicin, minocycline) [9]. This exploration includes various techniques, such as soaking, irrigation, coating, and impregnation. Various in vivo and in vitro studies show promising results [9].
Despite valuable insights provided by these experimental studies on the potential benefits of antimicrobial mesh treatment, there remains a scarcity of clinical studies that assess the effectiveness of this approach. This review aims to gather the current clinical evidence that is available and evaluate the impact of antimicrobial pretreated mesh on SSI in hernia repair surgery.
Methods
Search strategy and study selection
We report according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [10]. The study protocol is available on the PROSPERO database (CRD42023471619).
All clinical, published and unpublished studies investigating the effects of antimicrobial-treated mesh on SSI in adult human patients were eligible for inclusion. Only randomized controlled trials (RCTs) were considered for pooling in meta-analysis. Studies before the year 2000 were excluded, because they most likely did not utilize the most recent standards in perioperative clinical care, as described by Mangram et al. [11]. There was no restriction on language.
The Medline (PubMed), EMBASE (Ovid), CINAHL (EBSCO) and Cochrane Central Register of Controlled Trials (CENTRAL) databases were searched up to October 24, 2023. Key search terms included::“Hernia”, “Surgical procedures, Operative”, “Surgical Mesh”, “Anti-Bacterial Agents”, “Antiseptic”, “Infections” and “Surgical wound infection”. Any additional articles were unearthed through cross-referencing. The complete search strategy can be found in Online Resource 1. Full- text review and assessment were carried out when the title and abstract screening indicated the study eligibility.
Data collection and analysis
Data were extracted according to a pre-defined data abstraction form by two reviewers (NB and NH) independently. Study characteristics that were extracted included: study design, sample size, primary outcome, secondary outcome(s), route and type of agent used on mesh, mesh type, mesh location, type of surgery, wound classification as defined by the Centers for Disease Control and Prevention (CDC) [12]. Outcome data included incidence and definition of SSI (i.e., superficial, deep and organ space), hernia recurrence rate, follow up, reported pathogens and mesh infection. In case of missing data on SSI incidence, the corresponding authors were contacted.
The revised Cochrane risk of bias tool (RoB 2) was used to assess the risk of bias in the RCTs. Observational studies’ quality was judged with the Newcastle–Ottawa quality assessment form. Screening, data extraction, and bias/quality assessment were performed independently by two reviewers (NB and NH). Discrepancies were resolved through discussion.
Study characteristics are presented descriptively. If appropriate, outcome data were summarized in meta-analysis.
Relative risk (RR), corresponding 95% CI and standard errors were calculated for the individual comparative trial arms. Only RCTs with comparable administration of systemic antibiotic prophylaxis in either arm were pooled in the quantitative analysis, due to its strong effect on the primary outcome. Meta-analysis was performed using a random-effects model (Mantel-Haensel). A p value < 0.05 was considered statistically significant. Statistical heterogeneity was assessed using the I2.
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach was used for rating certainty of evidence using a minimally contextualized approach on the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias [13]. The minimally important difference was defined as 1.15% based on the default for appreciable benefit and harm of 25% and the SSI incidence of 4.6% in data of present meta-analysis of included RCTs for patients without antibiotic mesh treatment [14]. Inconsistency was assessed using I2 and τ2 statistics [15]. An I2 < 25% is considered as low, between 25 and 50% is considered moderate, and > 50% as high. We evaluated imprecision taking the minimally important differences into account. In case of large effects, the optimal information size approach was used by calculating the ratio of the upper to the lower boundary of the confidence interval with a threshold for downgrading of 2.5 [16].
Quantitative analyses were done using R, version 4.2.1 [R Core Team (2016) R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria], using the packages meta, metaphor and tidyverse.
Results
Study selection
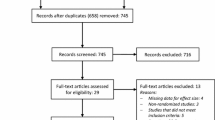
The PRISMA flowchart for study selection is shown in Fig. 1. Our search identified 1852 studies. After full-text screening of 18 articles, 11 were included in the systematic review. Reason for exclusion after full-text screening per article are presented in Online Resource 2.
Study characteristics
Study characteristics are presented in Table 1 and Online Resource 3. We included five RCTs [17,18,19,20,21], one comparative prospective study [22], one comparative retrospective study [23], three prospective cohort studies [24,25,26] and one retrospective cohort study [27]. Notably, two studies had three study arms each [18, 22]. Studies were published between 2001 and 2023. Seven studies investigated incisional or ventral hernia repairs and four studies describe patients undergoing inguinal hernia repair. All studies described the use of an antibiotic agent in the pretreatment of the mesh. Only one study by Schneeberger et al. 2020 [26] incorporated an antiseptic (povidone-iodine), in combination with antibiotics, for mesh pretreatment. No studies investigated the use of antimicrobial metals. Contamination levels varied.
Out of the five RCTs included in this review [17,18,19,20,21], four RCTs [17, 18, 20, 21] were pooled in meta-analysis on the efficacy of antibiotic mesh pretreatment on SSI reduction. Data were not included from a trial when systemic antibiotic prophylaxis was not used as a standard: one RCT administered systemic antibiotic prophylaxis exclusively in one group and only topical gentamicin on the mesh in the other arm [19]; similarly, one of the groups in the study by Seker et al. [18] received only topical gentamicin but no administration of systemic antibiotic prophylaxis.
Mesh treatment characteristics
A global overview of mesh type and treatment are presented in Table 1. A comprehensive description of the antimicrobial mesh treatment (i.e., concentrations of antimicrobials and specifics on topical application), location of mesh placement and systemic antibiotic prophylaxis are listed in Online Resource 4. Seven studies [17,18,19,20,21, 23, 26] used synthetic mesh, with six using polypropylene mesh and one only describing ‘a synthetic mesh’. Three studies [24, 25, 27] exclusively used biologic mesh. Fatula et al. [22] included patients treated with either biologic or synthetic mesh, wherein 3.8% of the patients were treated with a biologic mesh. Overall, 93.4% (2485/2660) of the total study population was treated with a polypropylene/synthetic mesh.
All of the meshes in intervention groups were treated with antibiotics; only one study combined antibiotics and an antiseptic [26]. Six studies [17,18,19,20, 22, 23] had an intervention arm wherein the mesh was treated with a singular antibiotic agent. Gentamicin was used in three RCTs and one observational study [17,18,19, 22]; the other two studies used vancomycin (RCT) [20] and rifampicin (observational) [23]. Mesh were treated with a combination of two antibiotics in one of the arms of five studies [21, 22, 24, 25, 27]; the combinations that were used comprised of gentamicin + clindamycin in one RCT and one observational study [21, 22], and rifampicin + minocycline [25, 27] or gentamicin + vancomycin [24] in observational studies. As mentioned before, the observational trial by Schneeberger et al. 2020 [26] not only treated the mesh with antibiotics but also used an antiseptic; in total, four antimicrobial agents were used for mesh treatment in that specific study (povidone-iodine, bacitracin, gentamicin, cefazolin).
In addition to the diversity of antimicrobials employed, topical mesh treatment techniques varied. The most common method was soaking of the mesh in antimicrobial solution before implantation (two RCTs, one observational study) [19, 20, 26]. Only the RCTs by Yabanoğlu [20] and Praveen [19] specified the time (15 min and 5 min, respectively) of soaking. One RCT and one observational study [18, 23] reported topical application of antibiotics on the mesh without further elaboration. Another RCT and observational study [21, 22] irrigated the mesh with antibiotic solution in the surgical field, maintaining a dwell time of 3 min maximum. Two observational studies [25, 27] used an antibiotic pre-coated surgical polypropylene mesh. In addition, two studies [17, 24] used an antibiotic carrier, which was implanted on top of the mesh before closing; calcium sulfate antibiotic beads with vancomycin + gentamicin (observational) [24] and an absorbable collagen tampon with gentamicin (RCT) [17].
Quality assessment
The assessment for risk of bias in the RCTs showed ‘some concerns’ for four studies [17, 19,20,21]. One study [18] was judged as having a high risk of bias. The observational studies were all rated as ‘poor’ with scores ranging from five to seven stars. The full Cochrane Risk of Bias assessment and Newcastle-Ottowa ranking are shown in Online Resource 5.
Data analysis
Across 11 studies involving 2660 patients, 196 SSI were reported leading to an overall incidence of 7.4%. Incidence ranged from 1.2% to 23.1% among studies. The meta-analysis of 1039 patients with 43 SSIs (4.1%) in the four RCTs comparing topical antibiotic mesh pretreatment with no antibiotic mesh pretreatment showed no significant reduction in SSI (RR 0.76 [95% CI 0.27; 2.09]). Only polypropylene meshes were included in the meta-analysis. With an I2 of 50% statistical heterogeneity was moderate. From all comparative (randomized or observational) studies, only the group from the study by Fatula et al. [22] using mesh pretreatment with both gentamicin and clindamycin solution showed a significant SSI reduction.
Data of the comparative observational studies were not pooled because of high heterogeneity. The forest plot for the meta-analysis of RCTs and non-pooled data of comparative observational studies is shown in Fig. 2.
Certainty of evidence
GRADE assessment, using a minimally contextualized approach, resulted in a very low certainty of evidence, as shown in Table 2. Since all included studies are RCTs, the starting certainty of evidence was high. There were no limitations regarding risk of bias since the result of the sensitivity analysis excluding high risk of bias studies was comparable to the main analysis. For inconsistency, we downgraded one level since heterogeneity was moderate (I2 = 50%). There was no indirectness [28]. We downgraded two levels for imprecision because the confidence interval overlapped thresholds of interest. For publication bias, rating down one level was necessary because the evidence consists of a number of small studies [29]. In total, we downgraded four levels resulting in a very low certainty of evidence. The full evaluation of certainty of evidence and considerations for grading is shown in Online Resource 6.
Secondary outcomes
All secondary outcomes are listed in Online Resource 3. Only five studies [19, 23,24,25, 27] reported hernia recurrence. Due to the lack of a control group in most of these studies, no quantitative analyses were performed. The same is true for mesh infection, which was only reported by two studies [25, 26] with three cases in total.
Three studies [19, 20, 23] reported on pathogens cultured from wounds. The most found bacterium was Staphylococcus aureus. The other bacteria identified in these studies were Enterobacter, Pseudomonas aeruginosa, Enterococcus faecalis, Proteus mirabilis, Escherichia coli and Staphylococcus epidermis.
Discussion
This systematic review and meta-analysis aimed to evaluate the effect of antimicrobial-treated mesh on SSI following hernia repair, offering new summary data on this topic. Analysis of 1039 patients with 43 SSIs from 4 randomized trials shows no significant benefit in SSI reduction for antibiotic mesh pretreatment when compared to no antibiotic mesh pretreatment. However, one observational study indicates a benefit of topical antibiotic mesh pretreatment on the risk of SSI.
SSI has the potential to develop into a mesh infection, one of the most detrimental complications of hernia repair [6]. Biofilm formation emerges as a key contributor to SSI and subsequent mesh infections [30], impairing host immune cells and impeding their ability to effectively combat and eliminate bacteria [31, 32]. In general, microorganisms exhibit a tendency to attach to surgical meshes, favoring rough, hydrophobic, and nutritional surfaces, such as polypropylene [31]. Nonetheless, in vitro studies have shown that biologic mesh might be more prone to bacterial adhesion than its synthetic counterpart [29, 31]. Considering the diverse array of available meshes, each differing in structure, composition, weight, porosity, absorbability, and other characteristics, it becomes evident that these variations significantly influence their susceptibility to infection. Therefore, exploring the influence of antimicrobial mesh pretreatment should be coupled with an understanding of the specific mesh type employed. This consideration is crucial when delving further into investigations regarding their collective impact on the risk of SSI and mesh infection.
Our review aimed to explore the clinical evidence regarding all types of antimicrobial mesh pretreatments for reducing SSI. Remarkably, all the studies we examined focused on the use of antibiotics as the primary antimicrobial agent. The sole exception was a study that investigated the topical application of povidone-iodine on mesh; however, even in this case, the antiseptic was combined with three types of antibiotics. Our search yielded no additional literature on the clinical application of antiseptic agents for this purpose.
Given the ongoing antibiotic resistance crisis [33], it is imperative to initiate clinical trials that investigate the potential efficacy of topical antiseptics and metals in the realm of mesh applications. Notably gentamicin, which is extensively used for mesh pretreatment in the included studies in this review, has been implicated in contributing to the escalating resistance observed among Staphylococcus species [34]. In contrast, both povidone-iodine and chlorhexidine as alternative agents considered for mesh pretreatment have not demonstrated a decline in bacterial sensitivity [35, 36].
Interestingly, the RCTs by Praveen [19] and Seker [18] found that topical antibiotic mesh pretreatment, in the absence of systemic prophylaxis, resulted in marginally superior outcomes for SSI compared to systemic antibiotic prophylaxis only. This observation raises considerations for inguinal hernia repair in clean settings, suggesting that the potential benefits of topical therapy may outweigh those of systemic approaches [37].
This review is limited by the lack of high-quality studies and significant clinical heterogeneity of available data. While all included studies incorporated antibiotic mesh pretreatment, substantial variations were observed in the types of antibiotics used, their modes of application, and exposure time. The type of mesh used differed among studies, for example synthetic or biologic mesh. Moreover, mesh was placed in various locations/layers of the abdomen. As mentioned, mesh type has its mesh-specific risk of (mesh) infection. The location for mesh placement is known to be associated with a location-specific risk of SSI and, for example, retro-muscular meshes have better mesh ingrowth [38]. These variables affect the risk of development of mesh infection and thereby the relative effect of mesh pretreatment. Some studies did not report a definition for SSI or worked with other definitions than those outlined in the CDC criteria [12]. In addition, the inclusion of all types of hernia surgery (both inguinal and ventral) introduces the limitation of data scattered across specific populations with varying SSI risk. However, we deem pooling SSI data from these repairs justified, since there is no plausible biological reasoning for effect modification between types of hernia surgery.
In light of the control group’s incidence in our meta-analysis (4.6%), a sample exceeding 10,000 patients would be necessary to demonstrate a clinically relevant 25% reduction in SSI. However, the RCTs included in our analysis, combining for only 1039 patients total, did not (adequately) describe their sample size calculation and are underpowered.
Conclusions
Data on topical mesh pretreatment to reduce SSI risk after hernia repair is limited. Very low certainty evidence from randomized trials in hernia surgery shows no significant benefit for antibiotic mesh pretreatment for SSI reduction, but data are imprecise due to optimal information size not being met. The diversity in mesh types, modes of antimicrobial agent delivery, and variations in reporting standards have contributed to a challenging landscape for drawing comprehensive conclusions.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lindenbergh KC, van Duinen AJ, Ahlbäck JG, Kamoh J, Bah S, Ashley T, Löfgren J, Grobusch MP, Sankoh O, Bolkan HA (2023) Prevalence, incidence, repair rate, and morbidity of groin hernias in Sierra Leone: cross-sectional household study. BJS Open. https://doi.org/10.1093/bjsopen/zrac158
Mavros MN, Athanasiou S, Alexiou VG, Mitsikostas PK, Peppas G, Falagas ME (2011) Risk factors for mesh‐related infections after hernia repair surgery: a meta‐analysis of cohort studies. World J Surg 35(11):2389–2398. https://doi.org/10.1007/s00268-011-1266-5
Baylón K, Rodríguez-Camarillo P, Elías-Zúñiga A, Díaz-Elizondo J, Gilkerson R, Lozano K (2017) Past, present and future of surgical meshes: a review. Membranes 7(3):47. https://doi.org/10.3390/membranes7030047
Mathes T, Walgenbach M, Siegel R (2016) Suture versus mesh repair in primary and incisional ventral hernias: a systematic review and meta-analysis. World J Surg 40(4):826–835. https://doi.org/10.1007/s00268-015-3311-2
Edmiston CE, McBain AJ, Roberts C, Leaper D (2015) Clinical and Microbiological Aspects of Biofilm-Associated Surgical Site Infections. In: Donelli G (ed) Biofilm-based Healthcare-associated Infections, vol I. Springer International Publishing, Cham, pp 47–67
Falagas ME, Kasiakou SK (2005) Mesh-related infections after hernia repair surgery. Clin Microbiol Infect 11(1):3–8. https://doi.org/10.1111/j.1469-0691.2004.01014.x
Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK et al (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 14(1):73–156. https://doi.org/10.1089/sur.2013.9999
Orelio CC, van Hessen C, Sanchez-Manuel FJ, Aufenacker TJ, Scholten RJPM (2020) Antibiotic prophylaxis for prevention of postoperative wound infection in adults undergoing open elective inguinal or femoral hernia repair. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003769.pub5
Mirel S, Pusta A, Moldovan M, Moldovan S (2022) Antimicrobial meshes for hernia repair: current progress and perspectives. J Clin Med 11(3):883. https://doi.org/10.3390/jcm11030883
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906. https://doi.org/10.1016/j.ijsu.2021.105906
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27(2):97–134. https://doi.org/10.1016/S0196-6553(99)70088-X
Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR et al (2017) Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg 152(8):784–791. https://doi.org/10.1001/jamasurg.2017.0904
Schünemann HB, Brożek J, Guyatt G, Oxman A (2013) GRADE Handbook for grading quality of evidence and strength of recommendations. Updated October 2013. https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 29 November 2023.
Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RAC, Santesso N, Traversy G et al (2021) GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings. J Clin Epidemiol 137:163–175. https://doi.org/10.1016/j.jclinepi.2021.03.026
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck-Ytter Y, Schünemann HJ (2011) GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 64(12):1294–1302. https://doi.org/10.1016/j.jclinepi.2011.03.017
Zeng L, Brignardello-Petersen R, Hultcrantz M, Mustafa RA, Murad MH, Iorio A et al (2022) GRADE Guidance 34: update on rating imprecision using a minimally contextualized approach. J Clin Epidemiol 150:216–224. https://doi.org/10.1016/j.jclinepi.2022.07.014
Musella M, Guido A, Musella S (2001) Collagen tampons as aminoglycoside carriers to reduce postoperative infection rate in prosthetic repair of groin hernias. Eur J Surg 167(2):130–132. https://doi.org/10.1080/110241501750070592
Seker D, Seker GE, Bayar B, Ergul Z, Kulacoglu H (2021) Topical antibiotic prophylaxis in Lichtenstein hernia repair and comparison of three methods: a prospective randomized clinical trial. Int. J. Abdom. Wall Hernia Surg. 4(2):58–63. https://doi.org/10.4103/ijawhs.ijawhs_6_21
Praveen S, Rohaizak M (2009) Local antibiotics are equivalent to intravenous antibiotics in the prevention of superficial wound infection in inguinal hernioplasty. Asian J Surg 32(1):59–63. https://doi.org/10.1016/S1015-9584(09)60011-7
Yabanoğlu H, Arer İM, Çalıskan K (2015) The effect of the use of synthetic mesh soaked in antibiotic solution on the rate of graft infection in ventral hernias: a prospective randomized study. Int Surg 100(6):1040–1047. https://doi.org/10.9738/INTSURG-D-14-00304.1
Warren JA, Lucas C, Beffa LR, Petro CC, Prabhu AS, Krpata DM et al (2024) Reducing the incidence of surgical site infection after ventral hernia repair: outcomes from the RINSE randomized control trial. Am J Surg. https://doi.org/10.1016/j.amjsurg.2024.01.004
Fatula LK, Nelson A, Hamza Abbad J, Ewing A, Hancock BH, Cobb WS, Carbonell AM, Warren JA (2018) Antibiotic irrigation of the surgical site decreases incidence of surgical site infection after open ventral hernia repair. Am Surg 84(7):1146–1151. https://doi.org/10.1177/000313481808400728
Kahramanca Ş, Kaya O, Azılı C, Celep B, Gökce E, Küçükpınar T (2013) Does topical rifampicin reduce the risk of surgical field infection in hernia repair? Ulus Cerrahi Derg 29(2):54–58. https://doi.org/10.5152/ucd.2013.35
Drohan A, Minor S (2020) Prospective study of single-stage repair of contaminated hernias with the novel use of calcium sulphate antibiotic beads in conjunction with biologic porcine submucosa tissue graft. Can J Surg 63(6):E530–E532. https://doi.org/10.1503/cjs.021819
Ilahi ON, Velmahos G, Janis JE, Kovach SJ 3rd, McLean SF, Askari R et al (2023) Prospective, multicenter study of antimicrobial-coated, noncrosslinked, acellular porcine dermal matrix (XenMatrix™ AB Surgical Graft) for hernia repair in all centers for disease control and prevention wound classes: 24-month follow-up cohort. Ann Med Surg 85(5):1571–1577. https://doi.org/10.1097/MS9.0000000000000695
Schneeberger SJ, Kraft CT, Janis JE (2020) No-touch technique of mesh placement in ventral hernia repair: minimizing postoperative mesh infections. Plast Reconstr Surg 145(5):1288–1291. https://doi.org/10.1097/PRS.0000000000006767
Baker EH, Lepere D, Lundgren MP, Greaney PJ, Ehrlich DA, Copit SE et al (2016) Early clinical outcomes of a novel antibiotic-coated, non-crosslinked porcine acellular dermal graft after complex abdominal wall reconstruction. J Am Coll Surg 223(4):581–586. https://doi.org/10.1016/j.jamcollsurg.2016.05.022
Zhang Y, Alonso-Coello P, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G et al (2019) GRADE guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences-risk of bias and indirectness. J Clin Epidemiol 111:94–104. https://doi.org/10.1016/j.jclinepi.2018.01.013
Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams JW, Meerpohl J, Norris SL, Akl EA, Schünemann HJ (2011) GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol 64(12):1277–1282. https://doi.org/10.1016/j.jclinepi.2011.01.011
Kathju S, Nistico L, Melton-Kreft R, Lasko LA, Stoodley P (2015) Direct demonstration of bacterial biofilms on prosthetic mesh after ventral herniorrhaphy. Surg Infect 16(1):45–53. https://doi.org/10.1089/sur.2014.026
Vitale C, Ma TM, Sim J, Altheim C, Martinez-Nieves E, Kadiyala U et al (2021) Staphylococcus epidermidis has growth phase dependent affinity for fibrinogen and resulting fibrin clot elasticity. Front Microbiol 12:649534. https://doi.org/10.3389/fmicb.2021.649534
Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms. Virulence 2(5):445–459. https://doi.org/10.4161/viru.2.5.17724
Ventola CL (2015) The antibiotic resistance crisis: Part 1: Causes and Threats. P&T 40(4):277–283
Gade Neeta D, QaziMohiuddin S (2014) Recent trend of aminoglycoside resistance among Staphylococcus aureus isolates in tertiary care hospital. JMA 6(6):94–96. https://doi.org/10.5897/JMA2014.0315
Aftab R, Dodhia VH, Jeanes C, Wade RG (2023) Bacterial sensitivity to chlorhexidine and povidone-iodine antiseptics over time: a systematic review and meta-analysis of human-derived data. Sci Rep 13(1):347. https://doi.org/10.1038/s41598-022-26658-1
Barakat NA, Rasmy SA, Hosny A, Kashef MT (2022) Effect of povidone-iodine and propanol-based mecetronium ethyl sulphate on antimicrobial resistance and virulence in Staphylococcus aureus. Antimicrob Resist Infect Control 11(1):139. https://doi.org/10.1186/s13756-022-01178-9
Ray P, Singh S, Gupta S (2019) Topical antimicrobial therapy: current status and challenges. Ind J Med Microbiol 37(3):299–308. https://doi.org/10.4103/ijmm.IJMM_19_443
Holihan JL, Nguyen DH, Nguyen MT, Mo J, Kao LS, Liang MK (2016) Mesh location in open ventral hernia repair: a systematic review and network meta-analysis. World J Surg 40(1):89–99. https://doi.org/10.1007/s00268-015-3252-9
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
NB and MAB were responsible for the conceptualisation. MAB supervised the project. NB, NJH, and MAB were actively involved in planning the methodology. NB and NJH contributed to the investigation, project administration, visualisation and writing of the original draft. NB and HG worked on data curation and formal analysis. AST, SWJ and MAB provided critical advice. NB and NJH accessed and verified the underlying data reported in the manuscript. All authors had full access to all the data and responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Conflict of interest
Author MAB reported receiving institutional grants from J&J/Ethicon and 3M; and being a speaker and/or instructor for J&J/Ethicon, 3M, BD, Gore, Smith & Nephew, TelaBio, Angiodynamics, GDM, Medtronic, Molnlycke. All other authors declare that they have no conflict of interest.
Ethical approval
No ethics committee approval was needed for this systematic review.
Human and animal rights
This article does not contain any studies with human participants or animals performed by the authors.
Informed consent
Not applicable.
Data transparency
NB and NJH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Other disclaimers
The information reported in the manuscript has not been presented previously.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bontekoning, N., Huizing, N.J., Timmer, A.S. et al. Topical antimicrobial treatment of mesh for the reduction of surgical site infections after hernia repair: a systematic review and meta-analysis. Hernia (2024). https://doi.org/10.1007/s10029-024-02987-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10029-024-02987-0