Abstract
Purpose
Giant inguinoscrotal hernia are a real challenge for every kind of surgeon. The technique that we adopt is suggested as a good option to deal with this cases. We report our experience in five cases of giant inguinoscrotal hernia with loss of domain from 2005 to 2012.
Method
Five patients with hernia that descended below the knees in the standing position, with an anteroposterior diameter not inferior to 30 cm and a laterolateral diameter of about 50 cm. Penis was not visible. We did the same procedure for all the five patients: single pararectus incision extended to groin region until proximal half of scrotum, isolation of the entire large sac out of the scrotal cavity, paying attention to not opening it, progressive reduction of the viscera without opening the sac with the hug technique, as shown in the video, placement of a heavyweight polypropylene meshes in the preperitoneal space, scrotal skin reductive plastic. In three of our five cases we obtained restoration of herniated viscera without resection of them. Orchiectomy was performed in all cases.
Results
No general neither wound complications were recorded. Long term follow up ranges from 8 years to 18 months: we did not record recurrence or chronic groin pain and scrotal size is normal in each patient.
Conclusion
The technique proposed permits to treat with success giant inguinaoscrotal hernia, avoiding the use of further specific procedure such as the preoperative progressive pneumoperitoneum. All our patients were satisfied with the surgeries and their quality of daily life had definitely improved.
Similar content being viewed by others
Introduction
Giant inguinoscrotal hernias have been defined as those that extend below the midpoint of the inner thigh with the patient in the standing position [1]. Giant inguinoscrotal hernias, with a significant secondary abdominal cavity, are infrequent in developed countries; nevertheless on rare occasions, patients visit their clinician after years of neglect and refusing to admit their problem. Even among underserved populations, the incidence of giant inguinoscrotal hernias is less than that of large inguinoscrotal hernias: indeed, this evidences the real distinction between giant and large inguinoscrotal hernias. In fact, giant inguinoscrotal hernias are not only those that extend below the midpoint of the inner thigh when the patient is standing, but also those with an anteroposterior diameter of at least 30 cm, a laterolateral diameter of about 50 cm and have been non reducible for more than 10 years.
The size of the hernia often causes difficulty in walking, sitting, or lying down. The penis is frequently buried inside the scrotum, causing urine to dribble over the already distended scrotal skin. This can lead to ulceration and secondary infection. Patients may also complain of difficulty in voiding [2]. Obviously other complications, such as intestinal obstruction and strangulation, are also possible, though rare.
Patients and method
We report five cases of giant inguinoscrotal hernia with loss of domain, from 2005 to 2012. The patients were 70, 60, 56, 52, and 38 years old. Except for their ages, they all presented a similar history, and all had relatively good health and complied with the presence of an initially reducible inguinal hernia, but had not undergone surgery because they had refused to accept their condition and feared surgery. Year by year, the hernias had enlarged significantly impacting the patients’ quality of life, including sexual function. The first three patients did not complain of comorbidities, the fourth patient was a smoker, and the last was obese and suffered from hypertension (Table 1). When asked why they had waited so many years before requesting surgery, their answers were similar; though they had initially feared the operation, this was eventually outweighed by the strange and unbearable feeling of a “space” between their legs and sexual difficulties.
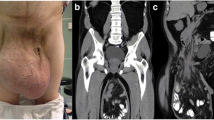
The clinical examination was similar for all five patients, a giant inguinoscrotal hernia that descended below the knees in the standing position with an anteroposterior diameter of at least 30 cm and a laterolateral diameter of about 50 cm. The penis was not visible, having been buried by the expanded scrotal sac, with only the urethral meatus apparent on examination. Peristaltic movement was clearly seen through the enlarged scrotal sac. The testes were impalpable (Figs. 1, 2).
Before surgery, we required, in addition to standard tests (complete blood count, chest X-ray, ECG), a spirometry, arterial blood gases, and a CT scan of the abdomen. We prepared the patients as we would have for a bowel operation, with a colon preparation. We administered cefamezin and metronidazole as antibiotic prophylaxsis at anesthesia induction. We scheduled recovery in the Intensive Care Unit for the initial postoperative period. Prior to surgery, each patient signed an informed consent, in which orchiectomy and bowel resection were included, in addition to standard surgical risks.
We employed the same procedure for all five patients, using the following steps:
-
1.
Single pararectus incision extending from the level of the umbilicus to the groin region and extending down the proximal half of scrotum.
-
2.
Isolation of the entire large sac from the scrotal cavity was performed, taking care not to open the sac (Figs. 3, 4). The testis was found to be hypotrophic and covered with scar tissue in all cases; as such, an orchiectomy was performed.
-
3.
Opening of the inguinal channel—this was achieved starting at the level of the umbilicus with an incision of the lateral margin of the anterior rectus sheet, extended inferiorly to the level of the external inguinal ring with opening of the external oblique aponeurosis. This pararectus incision included the medial insertions of the internal oblique muscle fascia to the rectus muscle fascia and, behind these, the deep portion of the transversalis fascia that was opened longitudinally. This separation of the lateral margin of the rectus muscle from the internal oblique muscle at the level of the umbilicus (Fig. 5) was continued distally to the internal inguinal ring and below it. At this level, the fibers of the internal oblique muscle were completely cut and the epigastric vessels separated and ligated (Figs. 6, 7). In this way, the entire internal ring was cleared, and a complete opening and communication between the posterior and anterior inguinal region was achieved, allowing the preperitoneal space to be approached widely. Practically speaking, the approach to the preperitoneum was achieved through a classical pararectus incision, completed with the section of the epigastric vessels and the internal ring. Just to remind, normally the internal ring is bounded, above and laterally, by the arched lower margin of the transversalis fascia and the inferior portion of the internal oblique muscle and below and medially, by the inferior epigastric vessels. It is important to understand that in the giant inguinal scrotal hernia, the anatomy and the anatomical structures of the internal ring are subverted as the fibers of the internal oblique muscle are pushed upwards and the anatomical separation between anterior and posterior inguinal region does not exist anymore.
Fig. 5 Drawing of the preperitoneal space achieved by pararectus incision The pararectus incision extending from the umbilicus through to the groin region and to the mid-scrotum with the separation between rectus muscle and oblique muscles. The internal oblique at the level of the internal ring and the epigastric vessels in this drawing are not yet separated
Fig. 6 -
4.
Progressive reduction of the viscera without opening the sac using the hug technique, as shown in the video (Online Resource 1 and Fig. 8), was utilized. The surgeon gently embraces the entire sac with his arms, feeling the abdominal cavity resistance being overcome and allowing the viscera to be gradually and completely reduced. This mechanism induces a slow, progressive, and continuous emptying of bowel content into the distal portion. In the first two cases, complete reduction was not possible, so the sac was opened, and a right hemicolectomy with a latero-lateral ileo-transverse colon anastomosis was performed to restore bowel continuity. In the last three cases, we were able to completely reduce the viscera into the abdominal cavity without resection; the sac reduction required about 1 h for each procedure.
-
5.
The space behind the rectus muscle, from the internal part of the pubic symphysis and the controlateral Cooper ligament to the umbilicus, was prepared. The Retzius space, the ipsilateral Cooper ligament, the iliac vein and artery in the Bogros space, the obturator region, and the psoas region were dissected. In this space, one approximately 30 × 30 cm heavyweight polypropylene mesh (Fig. 9; Table 2) was placed and fixed with non-absorbable sutures to the fibrous tissue of the internal pubic symphysis and to Coopers ligament (ipsilateral and controlateral). In addition, one absorbable suture was placed in the psoas muscle, and a non-absorbable transmuscular suture was placed in the rectus muscle. The choice of a heavyweight mesh is justified in all cases because of the totally destroyed posterior wall and the wide component separation needed to achieve a sufficiently large preperitoneal space. The mesh is spread in the preperitoneal region, as previously described, from the controlateral retropubic space to the ipsilateral psoas muscle region. The length of the mesh was approximately 30 cm because the extension of the mesh goes from below the umbilicus to the prevesical Retzius space (3–4 cm below the inferior edge of the pubic bone) and it is folded toward the retroperitoneal space in order to achieve a complete reinforcement of the visceral sac. Fibrin glue was sprayed on the entire mesh surface to better fix it to the wall and to reduce the risk of seroma after the wide dissection.
Table 2 Meshes placed Then a drain went from the space of Retzius through the opened posterior wall of the inguinal region, into the residual scrotal cavity and through the scrotal skin. The suction drain was left in place until the drainage diminishes to 20 cc a day.
-
6.
Abdominal wall closure—After the placement and fixation of the mesh, the purpose is being to achieve restoration of the posterior wall and the reconstruction of the opening between the internal oblique fascia and rectus fascia (Fig. 10). First the internal oblique fascia was reapproximated to the inguinal ligament to restore the posterior wall of the inguinal channel. Then the lateral edge of the rectus muscle was reapproximated to the medial edge of the internal oblique muscle. The next step was the closure from up to down of the anterior rectus sheath to the internal oblique fascia and finally the closure of the external oblique aponeurosis.
-
7.
Scrotal skin reductive plastic surgery: starting from the proximal scrotum, two longitudinal incisions were made continuing distally removing from each side 25 % of the excess skin and paying careful attention to hemostasis of the subcutaneous dartos. A running suture of all the subcutaneous tissue planes was placed from the distal to proximal scrotum, achieving a complete closure of all of the cavity and dead spaces. The skin was then closed with interrupted sutures or staples (Fig. 11).
The mean surgery time was 332 min (ranging from 310 to 360 min). The long duration of the procedure is justified by careful attention to all the previously mentioned steps; for example, in step 2, it is imperative not to open the sac, thus avoiding excessive bleeding in the scrotal cavity. Steps 3 and 5 essentially ensure a thorough preparation of the entire anatomic region to create sufficient space, for the large mesh and to provide enough tissue to cover it. In step 4, the crucial part of our hug technique must be performed with considerable patience, because a safe reduction can only be achieved by allowing enough time for the bowel and ileal loop to empty. Finally, the positioning of the mesh, its fixation, the reconstruction of the wall, and the scrotal skin plastic closure are all time-consuming steps if done carefully.
Patients were admitted to the Intensive Care Unit, where they were left on the ventilator for 24 h, during which their respiratory function was carefully monitored (Table 3). After extubation, all patients started respiratory therapy, and none experienced respiratory distress. Two days after surgery, all patients were discharged from the ICU. The patient with the bowel resection started liquids on the second postoperative day and had a bowel movement on the fourth postoperative day. The last patient, without bowel resection, started a liquid diet on the second day and had a stool on the third day. Antibiotic prophylaxis was administered for the entire hospitalization. Patients were able to return home after an average of 7.2 days (ranging from 6 to 9) (Table 4).
Results
No general or wound-related complications were recorded in the postoperative period for any of the patients. Long-term follow-up ranged from 18 months to 8 years: there were no hernia recurrence or chronic groin pain, and the scrotal size was normal in all patients (Fig. 12). The last patient reported a lumbar para-incisional hernia 1 year after surgery and is now on a waiting list for surgery. All patients were highly satisfied with the surgical procedures and their quality of life significantly improved.
Discussion
The surgical management of giant inguinoscrotal hernias can lead to potentially fatal complications [2] as the surgeon is faced with the problem of returning herniated viscera to the abdominal cavity after years of scrotal displacement. Precipitous reduction of hernia contents into the contracted peritoneal cavity may produce changes in intra-abdominal and intrathoracic pressure, potentially precipitating severe cardiac and/or respiratory failure, and a compartment syndrome [2–7]. Moreover, reduction under excessive tension places the patient at risk of wound breakdown, with the incidence of wound dehiscence and recurrence of the hernia reported in up to 30 % of patients [8].
The restoration of domain has been addressed by a number of techniques, most of which have originally been reported for the treatment of massive ventral hernias. The first option involves debulking the abdominal contents, i.e., performing an omentectomy, colectomy, or small-bowel resection [2, 6, 9]. Of course this technique facilitates visceral reduction, but can be complicated by peritoneal contamination with visceral and mesh infection [10]. We used this technique in the first two patients without complication. Probably our first two cases should be included in our learning curve; in fact in the remaining three cases, after years spent repairing complex abdominal wall hernias, we were able to reduce all herniated viscera by applying the hug technique and without the need for any kind of resection.
Another technique, described by Moss [4], uses an elemental diet as a means of reducing visceral volume by minimizing intestinal secretions and fecal volume. Although Moss described a decrease in visceral volume of approximately 2 L over a period of 1 month, the efficacy of this technique in extremely large hernias remains questionable.
Induction of preoperative progressive pneumoperitoneum to treat very large hernias with loss of domain was introduced in 1940 by Goňi Moreno [11]. It is usually recommended for giant ventral hernias, but rarely for giant inguinal hernias [12–18]. Preoperative progressive pneumoperitoneum (PPP) was recommended for patients with giant loss of domain hernias, including a large amount of viscera in the hernia sac. There were two reported indications for PPP: (1) when it would not otherwise be possible to perform the hernioplasty due to loss of domain and (2) when forced reduction of the hernia sac might cause the patient to develop the abdominal compartment syndrome postoperatively [19]. Preoperative progressive pneumoperitoneum increases the capacity of the retracted abdominal cavity, achieves a pneumatic lysis of intestinal adhesions, allows the reduction of the hernia contents, and improves diaphragmatic function. Preoperative progressive pneumoperitoneum also facilitates dissection of the hernia sac and can locate other hernias or weak zones that may not have been evident in the initial examination. Stretching of the hernia sac from PPP has been found to be helpful in skin cleansing before the operation and can potentially decrease the incidence of infections [14–16, 20]. Preoperative progressive Pneumoperitoneum is contraindicated in patients suffering from cardiac and pulmonary insufficiency and abdominal infections, and it requires a prolonged preoperative hospital stay that ranges from 7 to 18 days [13, 14, 21, 22].
On the other hand, some authors report technical failure of PPP, with air spreading into the hernia sac and only succeeding in expanding the sac with minimal effect on the contracted abdominal cavity [3, 5, 7, 23]. In a case reported by Vasiliadis [24], the PPP that begun 18 days before surgery did not allow the hernia content to be accommodated in the abdominal cavity, and a right hemicolectomy was necessary to reduce the remaining intestine. In light of these considerations and given our inexperience with PPP, we chose not to use it and were nevertheless able to restore the viscera to the abdomen.
At this point, it might be helpful to provide further clarification about the hug technique. We have emphasized the importance of not opening the sac throughout the procedure, based on two initial experiences (before the hug technique was introduced) in which opening of the sac was seen as unavoidable in order to achieve intestinal reduction. This was most likely because we did not have the patience to do the reduction properly. In fact in these cases, once the sac was opened, the huge amount of free jejunal/colonic bowel more or less clinging to one another and the sac, spread across the entire operating area, making the situation more and more difficult. Before resorting to a resection, we attempted decompression and reduction, but this proved to be practically impossible due to the constant escape of the other loops once some had been reduced. As such, this situation of incomplete reducibility made a resection necessary. In addition, if it is possible to avoid opening the sac, the length of paralytic ileus is less. Potential intra-hernia sac adhesions do not interfere with the hug maneuver. The key principle is that it is not necessary to detach the hernia content from the hernia sac, but to reduce the “volume” of the content inside the jejunal–colonic loops so that they can “collapse” and be slowly reduced into the cavity. At the end of the procedure, the entire sac is reduced with the content. It is important to emphasize that the decompression we achieve with the hug maneuver is highly effective and much simpler than all the viscera spread out on the operating field. The hug maneuver may seem difficult, and it can be if the surgeon is not patient. Our feeling is that if this step is correctly performed with enough patience, which may mean an additional hour of operative time, a resection can almost always be avoided. Only if the surgeon is not patient enough and does not feel the sensation of progressive emptying of bowel content he or she must proceed to open the sac and perform a resection.
Merret et al. [3] have managed a case of giant inguinal hernia by creating a midline anterior wall defect, covering both the hernia and the midline defect with Marlex mesh and strengthening the midline mesh using a rotation flap of the inguinoscrotal skin, which would otherwise have been discarded. Mehendal et al. [2] propose the reconstruction of the abdominal wall using Marlex mesh (12 × 25 cm), and in order to enhance the strength of the repair, they provide additional vascularized soft tissue cover using a tensor fascia lata pedicled flap raised from the thigh.
Ek et al. [25] propose the use of the grossly expanded peritoneum and redundant scrotal skin to increase the intra-abdominal capacity. A peritoneal flap was raised from the sac and reinforced using a polypropylene mesh with redundant scrotal skin fashioned into a myocutaneous flap to provide skin coverage. In a second-stage revision, the redundant scrotal skin was excised.
In our experience with the hug technique and the placement of a large, stiff, and heavy mesh, there is no need to create a midline anterior wall defect for the restoration of viscera in the abdomen or to make a rotation flap using the scrotal skin. By performing a scrotal reductive plastic operation and hernia repair at the same time, we avoid the need for a second operation and a period in which the patient has an excessive amount of redundant skin. In addition, our approach embraces the theory of the giant prosthesis for reinforcement of the visceral sac, proposed initially by Stoppa [26] and later by Wantz [27].
With regard to the incision, several approaches are described in the literature, in some cases involving multiple incisions. A standard transverse inguinal incision [10, 23, 24] or a midline laparotomy [9, 22, 25] does not allow complete reduction of the hernia contents. Valliattu et al. [22] propose for bilateral giant inguinoscrotal hernias an initial midline laparotomy to reduce the contents of the hernia sac into the peritoneal cavity and then they perform a bilateral abdominal muscle component separation to increase the flexibility of the abdominal wall and reduce the intra-abdominal pressure after closure. They also repair the hernias employing a preperitoneal approach through the same incision or through separate inguinal incisions.
We chose to use a pararectus incision extended through the groin region to the proximal half of the scrotum. This approach permits us to isolate the entire hernia sac from the scrotum (without opening the sac), and it affords an unobstructed view of the inguinal canal during the reduction of the hernia and easy access to the preperitoneal space, and during the mesh placement, a safe way to access the abdominal cavity in the event a resection is necessary.
Conclusion
The proposed technique makes it possible to successfully treat giant inguinal hernias with a single incision and a single operative procedure. It avoids the need for PPP and a prolonged preoperative hospital stay. In three of the five cases reported, we achieved restoration of the herniated viscera without a resection, as well as a positive cosmetic result. All of our patients were very satisfied with the surgery and their quality of daily life definitely improved. This procedure should be limited to centers specializing in abdominal wall repair with dedicated anesthesiologists and Intensive Care Unit physicians.
References
Hodgkinson DJ, McIlrath DC (1984) Scrotal reconstruction for giant inguinal hernias. Surg Clin N Am 64:301–313
Mehendale FV, Taams KO, Kingsnorth AN (2000) Repair of a giant inguinoscrotal hernia. Br J Plast Surg 53:525–529
Merrett ND, Waterworth MW, Green MF (1994) Repair of giant inguinoscrotal inguinal hernia using marlex mesh and scrotal skin flaps. Aust N Z J Surg 64:380–383
Moss G (1975) Techniques to aid in hernia repair complicated by the loss of domain. Surgery 78:408
Forrest J (1979) Repair of massive inguinal hernia. Arch Surg 114:1087–1088
Serpell JW, Polglase AL, Anstee EJ (1988) Giant inguinal hernia. Aust N Z J Surg 58:831–834
Kyle SM, Lovie MJ, Dowle CS (1990) Massive inguinal hernia. Br J Hosp Med 43:383–384
Stoppa RE (1989) The treatment of complicated groin and incisional hernias. World J Surg 13:545–554
Patsas A, Tsiaousis P, Papaziogas B, Koutelidakis I, Goula C, Atmatzidis K (2010) Repair of a giant inguinoscrotal hernia. Hernia 14:305–307
Monestiroli UM, Bondurri A, Gandini F (2007) Giant inguinoscrotal hernia. Tech Coloproctol 11:283–285
Goňi-Moreno I (1947) Chronic eventrations and large hernias: preoperative treatment by progressive pneumoperitoneum—original procedure. Surgery 22:945–953
Van Geffen HJ, Simmermacher RK (2005) Incisional hernia repair: abdominoplasty, tissue expansion, and methods of augmentation. World J Surg 29:1080–1085
Koontz AR, Graves JW (1954) Preoperative pneumoperitoneum as an aid in the handling of gigantic hernias. Ann Surg 140:759–762
Willis S, Schumpelick V (2000) Use of progressive pneumoperitoneum in the repair of giant hernias. Hernia 4:105–111
Mayagoitia JC, Suárez D, Arenas JC, Díaz de León V (2006) Preoperative progressive pneumoperitoneum in patients with abdominal-wall hernias. Hernia 10:213–217
Murr MM, Mason EE, Scott DH (1994) The use of pneumoperitoneum in the repair of giant hernias. Obes Surg 4:323–327
Toniato A, Pagetta C, Bernante P, Piotto A, Pelizzo MR (2002) Incisional hernia treatment with progressive pneumoperitoneum and retromuscular prosthetic hernioplasty. Langenbeck’s Arch Surg 387:246–248
Beitler JC, Gomes SM, Coelho AC, Manso JE (2009) Complex inguinal hernia repairs. Hernia 13:61–66
Munegato G, Grigoletto R, Brandolese R (2000) Respiratory mechanics in abdominal compartment syndrome and large incisional hernias of the abdominal wall. Hernia 4:282–285
Piskin T, Aydin C, Barut B et al (2010) Preoperative progressive pneumoperitoneum for giant inguinal hernias. Ann Saudi Med 30(4):317–320
El Saadi AS, Al Wadan AH, Hamerna S (2005) Approach to a giant inguinal hernia. Hernia 9:277–279
Valliattu AJ, Kingsnorth AN (2008) Single-stage repair of giant inguinoscrotal hernias using the abdominal wall component separation technique. Hernia 12:329–330
Kovachev LS, Paul AP, Chowdhary P et al (2010) Regarding extremely large inguinal hernias with a contribution of two cases. Hernia 14:193–197
Vasiliadis K, Knaebel HP, Djakovic N (2010) Challenging surgical management of a giant inguinoscrotal hernia: report of a case. Surg Today 40:684–687
Ek EW, Ek ET, Bingham R (2006) Component separation in the repair of a giant inguinoscrotal hernia. Ann J Surg 76:950–952
Stoppa RE, Rives JL, Warlaumont CR, Palot JP, Verhaeghe PJ, Delattre JF (1984) The use of Dacron in the repair of hernias of the groin. Surg Clin N Am 64:269–285
Wantz GE (1989) Giant prosthetic reinforcement of the visceral sac. Surg Gynecol Obstet 169(5):408–417
Conflict of interest
MC declares no conflict of interest. AB declares no conflict of interest. PB declares no conflict of interest. GC declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Video 1 caption The video shows the progressive reduction of the viscera without opening the sac when utilizing the hug technique.
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 15292 kb)
Rights and permissions
About this article
Cite this article
Cavalli, M., Biondi, A., Bruni, P.G. et al. Giant inguinal hernia: the challenging hug technique. Hernia 19, 775–783 (2015). https://doi.org/10.1007/s10029-014-1324-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-014-1324-7