Abstract
Some biological invasions can result in algae blooms in the nearshore of clear lakes. We studied if an invasive crayfish (Pacifastacus leniusculus) modified the biomass and community composition of benthic macroinvertebrates and therefore led to a trophic cascade resulting in increased periphyton biomass, elevated littoral primary productivity, and benthic algae bloom in a lake with remarkable transparency [Crater Lake, Oregon, USA]. After quantifying the changes in the spatial distribution of invasive crayfish over a 13-year period, we compared biomass and community composition of littoral–benthic macroinvertebrates, periphyton biovolume, community composition, nutrient limitation, and the development of benthic algae bloom in locations with high and low crayfish density. In addition, we determined if the alteration in community structure resulted in directional changes to gross primary production and ecosystem respiration. The extent of crayfish distribution along the shoreline of Crater Lake doubled over a 13-year period, leaving less than 20% of the shoreline free from crayfish. At high crayfish density sites, benthic macroinvertebrate biomass was 99% lower, and taxa richness was 50% lower than at low crayfish areas. High crayfish sites show tenfold greater periphyton biovolume, sixfold higher periphyton biomass (chlorophyll a), twofold higher metabolic productivity, and the presence of large filamentous algae (Cladophora sp.). The invasion of crayfish had negative consequences for a lake protected under the management of the USA National Park Service, with direct impacts on many levels of ecological organization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Invasive crayfish reduced the biomass and community composition of macroinvertebrates.
-
Sites with crayfish show greater periphyton biovolume, biomass, and productivity.
-
Crayfish presence favored a filamentous algae bloom in the nearshore of a clear lake.
Introduction
Lakes are spatially heterogeneous ecosystems with nearshore littoral and offshore pelagic habitats providing different metabolic pathways for autochthonous energy sources (Devlin and others 2016; Vadeboncoeur and others 2003; Vander Zanden and Vadeboncoeur 2020). Littoral–benthic periphyton often dominate nutrient cycling (Vadeboncoeur and Steinman 2002) and total lake production in clear mountain lakes (Sadro and others 2011, 2022a; b). Nearshore littoral habitats can be diverse in structure and production and present high trophic efficiencies passed through the invertebrate food web to fishes compared to the offshore habitat (Vander Zanden and others 2006, 2011). Despite higher trophic efficiencies, lake littoral habitats show lower resilience to changes in environmental conditions, as they are proximate to the land's edge and have shorter retention times of water, nutrients, and detritus than open water habitats (Peters and Lodge 2009; Scordo and others 2022a). Despite the importance of littoral habitats and their sensitivity to environmental changes, most monitoring and research programs continue to focus on the open water habitat (Cantonati and Lowe 2014; Vadeboncoeur and others 2014, 2021).
Globally there is increased interest in understanding why filamentous algal blooms (FABs) in nearshore littoral habitats of clear lakes are occurring more often in absence of changes in the offshore pelagic waters (Brothers and others 2016; Vadeboncoeur and others 2021). Changes in the biomass, behavior, and community composition of benthic macroinvertebrates due to the introduction of invasive species can be an important cause of algal blooms in lake nearshore areas (Vadeboncoeur and others 2021). Invasive crayfish introduced to freshwater ecosystems profoundly influence food webs and ecosystem functioning (Twardochleb and others 2013; Jackson and others 2014). Once crayfish establish, natural rates of spread can approach several km year−1 in lake and stream environments (Bernardo and others 2011; Messager and Olden, 2018). Invasive crayfish can significantly and fundamentally modify nutrient cycles and contribute to declines in biodiversity (Lodge and others 2012; Bjurström and others 2010), including localized and complete extinctions of native macrophytes (Dorn and Wojdak 2004; van der Wal and others 2013), benthic invertebrates (Nyström and others 1999; Bjurström and others 2010; Usio and others 2009), and fish (Fitzsimons and others 2007; Peay and others 2009). The negative effects of introduced crayfish may be especially severe in systems that lacked native crayfish or functionally similar organisms (Lodge and others 2000; Twardochleb and others 2013).
We analyze the trophic influences of invasive crayfish on the littoral habitat of a lake with high transparency (Crater Lake, Oregon, USA). Specifically, we quantified if these large, mobile consumers modified the biomass and community composition of smaller benthic macroinvertebrates and therefore led to a trophic cascade resulting in increased periphyton biomass, higher nearshore productivity, and a FAB. While native to some watersheds in western North America (Larson and others 2012), non-native signal crayfish (Pacifastacus leniusculus) were introduced into Crater Lake in 1915 as food for non-native trout and salmon (Girdner and others 2018). The signal crayfish is considered one of the 100 world’s worst invasive species and is a major threat to freshwater biodiversity and ecosystem functioning (Lowe and others 2000; Ooue and others 2019). In Crater Lake, the establishment and movement of signal crayfish in the last decade has led to near extinction of a unique population of newts (Taricha granulosa mazamae; Girdner and others 2018). However, it is unknown if Pacifastacus leniusculus generates other ecological changes in the littoral habitat of this unique lake which may impact food resources for native newts, yield changes to the relative amount of ecosystem metabolic production between the littoral and pelagic habitats, or result in large-scale water quality changes in an otherwise well-documented ecosystem with high water quality conditions. For example, the first-ever documented FAB in the nearshore littoral habitats of Crater Lake occurred in 2021. We suggest that the bloom might have been favored by crayfish feeding on benthic grazers and thus reducing grazing pressure on filamentous algae.
Material and Methods
Crater Lake is a large (53 km2), ultraoligotrophic, subalpine (1883 m a.s.l.) lake located within Crater Lake National Park in southwestern Oregon, USA (Figure 1). The lake lies within a closed basin that occupies the caldera of the former Mt. Mazama (Bacon 1983) and is recognized for its depth (594 m maximum depth), high transparency (Hargreaves and others 2007), and lack of ice cover during winter months (Buktenica and others 2007). The littoral habitat substrate contains mostly cobble and boulder (Bacon and others 2002) and is limited by the steep slope (> 30°), which drops quickly to extremely deep water. A prominent feature within the lake that creates littoral habitat occurs at Wizard Island, the largest island in the lake (Figure 1). Surface water temperature ranges 2–18 °C, with maximum temperatures during July–August. Across the littoral habitat of the lake, temperature does not differ (Supplementary Material 1). Changes in climate have led to long-term changes in thermal structure (Stetler and others 2021), including warmer surface temperature during summer (0.6 C per decade since 1965), earlier onset of summer stratification (− 0.6 days per decade since 1966), and shoaling of summer thermocline depth (− 2.4 m per decade since 1993). Summer stratification usually begins in May with mid-summer thermocline depth typically around 10 m. The lake has low pelagic primary productivity (less than 0.7 mg C m–3 h–1) with peak chlorophyll a concentration less than 2 µg l−1 (McIntire and others 2007), an extremely deep chlorophyll maximum during summer (120 m; Girdner and others 2020) and summer Secchi depth typically between 25 and 35 m (Larson and others 2007). Although Crater Lake was naturally fishless, salmon and trout were introduced beginning in 1888 and currently persist as naturally reproducing populations of rainbow trout (Oncorhynchus mykiss) and kokanee salmon (Oncorhynchus nerka) (Buktenica and others 2007). Signal crayfish were introduced to Crater Lake in 1915 but spread very slowly for the first ~ 70 years. Horizontal crayfish movements along the shoreline have increased over the last ~ 20 years as summer water temperature and length of summer stratification have increased (Girdner and others 2018).
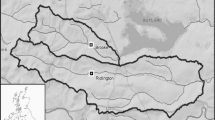
Sampling sites in areas with high (green) and low (blue) crayfish density in Crater Lake (Oregon, USA). We surveyed crayfish in 40 sites along the littoral habitat of the lake (red dots). We sampled macroinvertebrates in five sites (yellow dots), and periphyton in eight sites (light blue dots). We determined littoral metabolism and conducted experiments to quantify the influence of crayfish on biomass, nutrient limitation in one site with high crayfish density and one with low crayfish density (white dots).
We conducted time-constrained snorkel surveys in 2008 and 2012–2021 to assess spatial distribution and relative abundance of crayfish along the shoreline of Crater Lake. Locations for sampling were laid out systematically at 1000 m spacing around the shoreline. Sites were numbered sequentially beginning on the west side of the lake and increasing around the lakeshore in a clockwise direction (sites 200–264; Figure 1). Additional sites 266–276 were on the shore of Wizard Island (Figure 1). Snorkel surveys were conducted annually in late July or August at the 40 evenly numbered locations around the lake shoreline, including Wizard Island (Figure 1). At each survey location, two snorkelers swam in opposite directions along the shoreline turning over rocks and recording the total count of crayfish observed in a 10-min period.
We analyzed the impact of crayfish on the biomass and community composition of littoral–benthic macroinvertebrates. Samples were collected during summer with SCUBA divers who laid a square-meter quadrat over hard substrate and utilized a submersible battery-powered vacuum pump to collect and filter the benthos through a 500-µm mesh bag. The divers moved the intake hose of the vacuum pump over and under the rocky substrate being careful to sample all available surfaces within the square-meter area. Samples were then preserved in 70% ethanol and later sorted, counted, and identified to family. We collected two replicate macroinvertebrate samples at 3 and 10 m in the year 2009, 2011, 2014, 2015, and 2016. Additional samples were collected in 2019 and 2020 but time constraints limited sampling to 3 m depth. We collected a total of 55 macroinvertebrate samples at sites 222, 240, 246, 258, and 260 (Figure 1). Some of these sites never had crayfish (258 and 260), others did not have crayfish initially but were subsequently colonized (240–246), and site 222 had crayfish present prior to the first survey in 2009. Because crayfish spread spatially along the shoreline over the 13-year study period, we pooled the macroinvertebrate data for all sites and years and compared the biomass, richness, and community composition of the invertebrates based on crayfish density at a site at the time of sampling, classifying sites as either ‘low’ or ‘high’ crayfish density. Low crayfish density were all sites with snorkel counts equal or less than 1 crayfish (N = 19 samples). High crayfish density were sites with more than 50 crayfish (N = 36 samples). Most sites with low crayfish density had no crayfish observed during that snorkel survey but one crayfish was observed in some years just as crayfish were reaching an area. Several sites (240–246) transitioned from low to high crayfish density over the study period as crayfish spread along the shoreline and increased in density through time.
We compare periphyton biovolume and community composition (sampled in August 2020), periphyton nutrient limitation (an experiment in August 2021), littoral metabolism (summer 2018 and 2019), and the development of a FAB (summer of 2021), in locations with high (> 50) and low (≤ 1) crayfish density. Periphyton biovolume and community composition was determined from one rock at each of four sites with high crayfish density (210, 222, 232, and 242) and low crayfish density (202, 204, 250, and 252). Biofilms were removed from the entire rock surface using a brush and distilled water. An aliquot of each sample was preserved in Lugol’s solution and analyzed per USGS National Water-Quality Assessment periphyton protocols (Charles and others 2002) to the species level for both diatom and soft bodied algae by a commercial laboratory (Rhithron Associates). We calculated the upward facing surface area of each rock using a flatbed scanner and area calculator software (Microsoft Sketchandcalc). Results included cell density (cells cm−2) and cell biovolume (µm3 cm−2).
To quantify the influence of crayfish on periphyton biomass, we used two approaches. First, we conducted a reciprocal transplant experiment where eight rocks from a site with low crayfish density (252) were placed at a site with high crayfish density (222) and vice versa. The experiment was carried out for nine weeks during the summer from June 28 to September 1, 2021. In addition to the transplanted rocks, we collected eight rocks from each location that occurred naturally, representing the ambient conditions prior to and during the experiment. Periphyton biomass was determined for each transplanted and native rock by quantifying the pheophytin-corrected chlorophyll a concentration by surface area (µg cm −2) following the methods in Tromboni and others (2017). Chlorophyll was determined by methanol extraction and samples were analyzed on a Turner 10-AU fluorometer (Marker 1972). To calculate rock surface area with algae, we covered that area with foil and then calculated the extent of foil using the software ImageJ (Schneider and others 2012).
Second, we calculated littoral metabolism at a site with high crayfish density (site 222) and a site with low crayfish density (site 252) over two years. We used free-water, high-frequency observations of in situ dissolved oxygen obtained at 10-min intervals with PME miniDOT sensors located on nearshore moorings at both locations. The moorings consisted of stainless-steel wire rope anchored to the bottom at 7 m depth with a subsurface float approx. 2 m below the surface. The oxygen sensor and wiper were attached to the wire rope 3 m below the surface (1 m below the subsurface float). We estimated metabolism using a modeling approach based on the work Lottig and others (2022). This model requires high-frequency measurements of in situ dissolved oxygen concentration (mg l−1) and water temperature (°C), as well as meteorological measurements of incident photosynthetically active radiation (PAR; µmol m−2 s−1), wind speed (m s−1), and barometric pressure (mbar). Wind speed was collected from a buoy in the middle of Crater Lake at a height of 2-m above water surface at 1-h intervals. Hourly values for solar radiation and pressure were collected at a weather station on the crater rim on the south side of the lake. The metabolism model generates daily estimates (mmol O2 m−2 d−1) of gross primary production (GPP), ecosystem respiration (R), and net ecosystem production (NEP = GPP − R). The model structure is available in our repository (code o2_model_inhibition), and a detailed description of the metabolism model can be found in Lottig and others (2022). Metabolism estimates where calculated in 2018 and 2019 from July 15th to September 30th.
We deployed in situ nutrient-diffusing substrata (NDS) to determine nutrient limitation of periphyton at a site with high crayfish density (222) and a site with low crayfish density (252). NDS were designed using methods described in Tank and others (2017). Individual NDS cups were constructed using 30 ml clear polystyrene cups filled with 2% microbiology-grade agar by weight (Millipore Sigma). Control treatments contained only agar, while nutrient enriched cups (PO4, NH4, NO3, NO3 + PO4, NH4 + PO4) had 0.1 M KH2PO4, 0.5 M NH4Cl, 0.5 M KNO3, 0.1 M KH2PO4 + 0.5 M NH4Cl, and 0.1 M KH2PO4 + 0.5 M KNO3 added, respectively. We deployed 5 replicates per treatment in each location. Agar was topped with porous glass fritters (2.6 cm diameter, LECO Corporation) and subsequently sealed using screw-on polypropylene caps with a bored 2.2 cm diameter hole to allow epiphytic algal colonization and nutrient diffusion. NDS cups were zip-tied to L-shaped vinyl bars. L-bars were attached to a cinder block. We deployed the blocks at 1.5 m depth at each site to withstand wave action during incubation. The NDS experiments incubated for 21 days. Immediately after retrieval, we removed substrata from NDS cups and froze them for chlorophyll a analyses. Each glass fritter was extracted in 20 ml methanol for 24 h while refrigerated in the dark. Then, we measured pheophytin-corrected chlorophyll on a Turner 10-AU fluorometer (Marker 1972) which is presented in concentration of µg cm−2. We used the hierarchical logic rules from Maberly and others (2002) to determine the condition of nutrient limitation for this period: 1. P > C and N > C, both nutrients limiting; 2. P > C, P limitation, 3. N > C, N limitation, 4. NP > N or NP > P, Co-limitation; 5. P ≤ C and N ≤ C and NP ≤ N and NP ≤ P, no limitation. We did not find statistical differences between treatments amended with NO3 and NH4 (Supplementary Material 2); therefore, we combined the results of treatments with these nutrient additions as N.
An unprecedented FAB formed along many areas of the lake shoreline in mid-July 2021. This has never been observed in the long-term (40 years) monitoring record of Crater Lake. We measured density of the FAB at six locations spread spatially around the lake on July 29, 2021, including four sites with high crayfish density (222, 232, 242, and 270 on Wizard Island) and two sites with low crayfish density (204 and 252). We established 30 m long transects at the water line at each location. Five random locations between 0 and 30 m were determined. A one-meter square PVC frame was placed at each random location and the percent coverage of the filamentous algae within that frame was estimated. We also recorded the spatial coverage of sand, gravel, cobble, and boulder substrate within the frame. Substrate was similar between all sites (> 70% hard substrate, little sand and gravel). Percent coverage of the transect was the mean of the 5 measurements.
Differences among sites with high and low crayfish density for benthic invertebrate biomass and richness, periphyton biovolume, chlorophyll a, GPP, R, and NEP were quantified using linear mixed-effects models (LMM), with crayfish density (low or high) modeled as a fixed effect. When data were available for more than one year, we modeled the year as a random effect. Nutrient limitation was tested for each site independently using a LMM with nutrient modeled as a fixed effect. We considered the specific covariance structure for repeated-measures data in each model using the correlation argument in gls (or lme in cases model has random effects) function in the nlme R package (Pinheiro and others 2020). Also, when models did not meet the assumption of homoscedasticity, we modeled the variance using the weights argument in gls (or lme) function in the nlme R package. Tests for significance of the fixed effects in the models were performed via the Wald statistic (Zuur and others 2009) using the gls and lme functions in the nlme R package. Multiple comparisons among nutrients were performed with Tukey's HSD post hoc test (emmeans R package; Lenth 2021). We ran a permutational analysis of variance (PERMANOVA) using the adonis function in the vegan R package (Anderson and others 2006; Oksanen and others 2019) to compare the macroinvertebrate and periphyton community composition at sites with high and low crayfish density. All the analyses were performed in the statistical software R (R Core Team 2020). Data and metadata for this study are available at Scordo and others (2022b).
Results
Crayfish Distribution
The extent of crayfish distribution around the littoral–benthic habitat of Crater Lake has increased since 2008 (Figure 2). In 2008 there was crayfish at 14 of the 40 survey sites, and only 7 of those sites recorded more than 50 crayfish during the time-constrained survey. By 2021, there were crayfish at 32 of the 40 sites and 20 of those had more than 50 individuals (Figure 2).
Benthic Macroinvertebrates
Littoral sites with high crayfish density differed in benthic macroinvertebrate biomass, richness, and community composition from sites with low crayfish density. The biomass of macroinvertebrates was 99% lower (p < 0.01) at sites with high crayfish density (17 ± 3 g m−2) than at sites with low crayfish density (1414 ± 229 g m−2; Figure 3A). Also, taxa richness was 50% lower (p < 0.01) at high crayfish sites (5 ± 1 number of taxa) compared to low crayfish sites (11 ± 1 number of taxa; Figure 3B). Community composition at sites with high and low crayfish density was different (PERMANOVA, p < 0.01; Figure 3C). Snails and caddisflies represented 78% and 18%, respectively, of total biomass of macroinvertebrates at sites with low crayfish populations (≤ 1 crayfish). Snails (23% of total biomass) and caddisflies (4% of total biomass) were substantially reduced at sites with an established crayfish population (> 50 crayfish), while worms, amphipods, mayflies, and midges represented a higher percentage of total biomass at low crayfish sites (Figure 3C). The biomass of main grazers (snails) decreased by 99% from 1100 g m−2 at sites with low crayfish density to 4 g m−2 at sites with high crayfish density. For example, at site 240 the biomass of macroinvertebrates decreased from near ~ 1000 g m−2 before the crayfish invasion (2012) to ~ 10 g m−2 after two years of stablished crayfish population (2016; Figure 3D). At site 222 which had high crayfish density during the entire study period the macroinvertebrate biomass was 16 ± 5 g m−2 (Figure 3D). In contrast, site 258, which had low crayfish density the macroinvertebrate biomass was 895 ± 312 g m−2 (Figure 3D).
Comparison of benthic macroinvertebrate indicators at sites with high and low crayfish density. A biomass and B richness declines with high crayfish density while (C) and there is a loss of snail grazers. D Time series of macroinvertebrates biomass and crayfish population at site 240 (low crayfish density up to 2012, then high crayfish density), 222 (high crayfish density since 2008), and 258 (low crayfish density since 2008) in Crater Lake. At site 240 we observed a decrease in macroinvertebrate biomass as crayfish become established. In panel A boxplots has own y-axis with different scales as the biomass of macroinvertebrates was 99% lower at high crayfish sites than at low crayfish sites.
Periphyton
Periphyton biomass, community composition, and nutrient limitation were different in littoral–benthic sites with high crayfish density versus sites with low crayfish density. The biovolume of periphyton was tenfold higher (p < 0.01) at high crayfish sites (6.8*109 ± 0.9*109µm3 cm−2) compared to low crayfish sites (0.5*109 ± 0.3*109µm3 cm−2; Figure 4A). Community composition at sites with high and low crayfish density was similar (PERMANOVA, p = 0.11). However, we found Cladophora sp. only at sites highly impacted by crayfish (18% of total periphyton biomass; Figure 4B). Also, Epithemia turgida, the largest size species from this genus in Crater Lake, represented 38% and 11% of total periphyton biomass in the sites with high and low crayfish density, respectively (Figure 4B).
Differences in periphyton indicators between sites with an established population of crayfish compared to locations low crayfish presence. Sites with high crayfish density have tenfold greater biovolume (A), a periphyton community composition with larger and filamentous species (B), up to sixfold higher chlorophyll a concentration (C), twofold greater gross primary production and respiration compared with low crayfish sites (D). Panel C corresponds to the results of the reciprocal transplant experiment, where eight rocks from a site with high crayfish density were placed at a site with low crayfish density and vice versa, during nine weeks. The Panel C x-axis tiers show if the rocks where native from a site with low or a high crayfish density (origin), and if the rocks were placed in a site with low or a high crayfish density during the experiment (destiny).
Periphyton biomass measured as chlorophyll a was sixfold higher at sites with high crayfish density (9.7 ± 1.9 µg cm−2) than at sites with low crayfish density (1.5 ± 0.4 µg cm−2; p < 0.01, Figure 4C). The reciprocal transplant experiment showed that even two months of exposure to a site with high crayfish density was enough to produce changes in the concentration of chlorophyll a (Figure 4C). After the nine week experiment, rocks that were moved from the site with low crayfish density to the site with an established population of crayfish presented sixfold more biomass of periphyton chlorophyll a (11.2 ± 1.3 µg cm−2) compared to rocks in areas with low crayfish density (chlorophyll a = 1.8 ± 0.6 µg cm−2; Figure 4C).
The littoral site with high crayfish density had substantially higher metabolism (GPP and R) rates than the low crayfish site (p < 0.01; Figure 4D). We observed consistent differences between the sites in two years (2018 and 2019; Figure 4D). GPP ranged from 45.2 to 13.6 mmol O2 m−2 d−1 in the site with high crayfish density, and 23.2 to 5.6 mmol O2 m−2 d−1 in the site with low crayfish density. Values of R ranged from − 55.8 to − 38.6 mmol O2 m−2 d−1 at the high crayfish site, and − 26.5 to − 16.5 mmol O2 m−2 d−1 in the low crayfish site (Figure 4D). Our results show productivity can double in a location with high crayfish density when compared to a site with low crayfish density.
The nutrient limitation bioassays for periphyton indicated sites with high and low crayfish population are both limited by N and P, but the area with high crayfish density has more of an N deficit (Figure 5A). At the site with low crayfish density, additions of N + P, N, and P produced a significant change in chlorophyll a when compared to the control (p < 0.01) without a significant difference between treatments (p > 0.7; Figure 5A). Chlorophyll a at sites with high crayfish population was higher in the treatments where N + P, N, and P were added when compared to the control (p < 0.01; Figure 5A). Also, at sites with high crayfish density, chlorophyll a was higher when N + P and N were added when compared to the treatment where P was added (p < 0.01).
Periphyton in areas with high and low crayfish density are limited by N and P, but the site with high crayfish population has more of an N deficit (A). Treatment with different letter present significant differences in panel A. Photographs of unprecedented filamentous algae bloom along the shoreline of Crater Lake during the summer of 2021 due to an increase in temperature, which tended to be higher at sites with high crayfish density (B, C).
Filamentous Algal Bloom (FAB) Spatial Coverage
An unprecedented FAB occurred along the shoreline of Crater Lake during summer 2021, when large areas of the shoreline turned bright green due to the proliferation of filamentous green algae Cladophora (Figure 5C). This first-ever documented FAB in Crater Lake was associated with much warmer air temperature and record high surface water temperatures during June and July compared to the historical period (Supplementary Material 3). No other known drivers of algae growth (nutrient flux, precipitation, runoff, incident light) were substantially different in Crater Lake during 2021 compared to previous years (NPS unpublished data). The FAB did not occur along the entire shoreline of Crater Lake but tended to be higher along the north and eastern shores, as well as all of Wizard Island (Figure 5B, C), similar to the distribution of crayfish. Percent coverage of FAB at the sites with low crayfish density was low (mean 6.5%), whereas shoreline coverage at sites with an established crayfish population was high (mean 70%).
Discussion
Since 2008, non-native crayfish distribution along the shoreline of Crater Lake has nearly doubled, leaving less than 20% of the littoral–benthic habitat free from crayfish. Once crayfish became established in an area, macroinvertebrate grazers were reduced by 99%, resulting in an apparent trophic cascade between grazers and primary producers. We show that the drop in grazers led to increased periphyton biomass, elevated primary productivity of the benthos, and promotion of filamentous algae in this highly pristine lake. Our results show a change toward more periphyton biovolume and a community trajectory toward larger and filamentous species at sites highly impacted by crayfish. This study is unique in that the presence of an established crayfish population was also correlated with an increase in the overall nearshore algal productivity as measured via high-frequency dissolved oxygen sensors. Littoral productivity is an important ecosystem trait not often measured in lakes.
We developed a conceptual model of the trophic cascade resulting from crayfish introduction in Crater Lake (Figure 6). Sites with high crayfish density show less benthic macroinvertebrate biomass and richness and a massive reduction in grazers (snails). Consequently, sites with high crayfish population show tenfold greater periphyton biovolume, sixfold higher periphyton biomass (chlorophyll a), twofold higher periphyton primary productivity, and the presence of large filamentous algae (Cladophora). Furthermore, Girdner and others (2018) showed that the crayfish introduction led to a near extinction of a unique population of newts (Taricha granulosa mazamae) in this lake. These findings indicate that in less than 13 years, the spread of a long-lived (10 years; Momot 1984) cold water crayfish had deleterious consequences for a lake already protected within a USA National Park. The consequences are far reaching leading to direct changes in the ecosystem’s littoral food web, including loss of the native top predator (newt), reduced biodiversity of secondary consumers and reduced grazing pressure, as well as encouraging blooms of filamentous algae, an increasingly common issue observed recently in the world’s so-called pristine lakes (Vadeboncoeur and others 2021).
Conceptual model of the crayfish cascade effect on the ecology of Crater Lake littoral–benthic habitat. Crayfish have high predatory pressure on benthic macroinvertebrates reducing their biomass and richness with a substantial decrease in relative amount of snail grazers. The reduction in benthic macroinvertebrates releases grazing pressure on periphyton biofilms, increasing attached algae biomass and productivity, and favoring the appearance of filamentous algae.
Although crayfish were introduced into Crater Lake in 1915, horizontal movement along the shoreline has increased in recent decades, likely due to warmer water temperature and longer periods of summer stratification (Girdner and others 2018). The lateral movement of crayfish along the shoreline of Crater Lake since 2008 is faster (between 666 and 1300 m year−1) than at the beginning of the century (85 m year−1; Girdner and others 2018). Currently, only the southwest shoreline lacks invasive crayfish and continued warming is likely to allow crayfish to spread to the entire shoreline within 2–5 years. The progression of crayfish movement around the littoral–benthic habitat of Crater Lake is consistent with the “sleeper invader” phenomenon (Spear and others 2021). A "sleeper invader" is a non-native species that can be present in an ecosystem for a long duration before some other environmental change allows it to spread or impact as an invasive species.
Crayfish decreased the biomass and richness of benthic macroinvertebrates in Crater Lake, with snails, and caddisflies significantly reduced or eliminated at high crayfish locations. A marked reduction of benthic invertebrate biomass and diversity is typical of crayfish invasions (Nyström and others 1999; Lodge and others 2012), particularly in ecosystems that lack native predator species (Twardochleb and others 2013; Girdner and others 2018). The relative abundance of slow-moving benthic organisms (such as snails) decreases due to crayfish introductions while the proportion of mobile predatory invertebrates increases (Bjurström and others 2010; Ruokonen and others 2014; Ercoli and others 2015). Conversely, snail density and taxa richness can increase following a decline in crayfish density (Hansen and others 2013; Ruokonen and others 2016).
With a reduction of zoobenthic biomass and functional feeding groups (for example, grazers), we observed a concomitant increase in the benthic biofilms and overall nearshore benthic primary productivity. A few studies have shown high crayfish density enhanced the biomass of periphyton through positive cascade effects (Weber and Lodge 1990; Lodge and others 1994; Nyström and others 1999, 2001) or via bioturbation from crayfish physical activity (Charlebois and Lamberti 1996; Stenroth and Nyström 2003) by keeping stone surfaces free of surface deposits (Whitmore 1997). However, as crayfish biomass replaces other benthic macroinvertebrate biomass, one may not expect a trophic cascade, because crayfish can also feed on periphyton or algae (Twardochleb and others 2013). Twardochleb and others (2013) showed weak or non-existent effects of most invasive crayfishes (including signal crayfish) on periphyton despite crayfish decreasing benthic macroinvertebrate biomass, because crayfish can also feed on algae. Ludlam and others (2015) found little evidence for a crayfish trophic cascade in mesocosm experiments, because crayfish also fed on the periphyton. Similarly, Usio (2000) found that crayfish did not produce a trophic cascade in detrital leaf packs when they consume shredding invertebrates because crayfish themselves also consumed the detritus. Furthermore, in systems with algal communities having filamentous forms (that is, Cladophora), crayfish grazing on filaments may be significant (Creed 1994) and may outweigh reduced grazing from snails (Nyström and others 1996).
We present several hypotheses which may favor a positive crayfish driven trophic cascade on periphyton algae in Crater Lake, in addition to the spatial association of crayfish with the distribution of the first-ever FAB in 2021. We speculate that the correlation of crayfish with an increase in periphyton algae may result from the extreme nutrient limitation in Crater Lake combined with differences in feeding morphology between crayfish and snail grazers. Crater Lake is extremely nutrient poor. For example, dissolved nitrate is less than 0.008 mg l−1 within the summer photic zone (Larson and others 2007), mean summer Secchi disk depth is greater than 30 m (Girdner and others 2020), and depth of 1% visible light penetration in Crater Lake is typically 90–100 m (Hargreaves and others 2007). The extreme nutrient limitation allows native benthic invertebrate grazers (snails primarily) to keep periphyton at an extremely low level under most natural conditions, (chlorophyll a ~ 1.5 μg cm−2). Because crayfish due to their feeding morphology do not graze as efficiently on periphyton as do snails (Luttenton and others 1998), the much larger signal crayfish may not be able to keep algae at such a low level even if feeding on periphyton following the reduction of invertebrate grazers. Comparing bulk carbon and nitrogen stable isotope values of crayfish at locations with and without benthic invertebrates could be used to investigate the niche of each species and the degree to which signal crayfish consume periphyton algae.
Although shoreline FABs are commonly associated with cultural eutrophication (Dodds and Gudder 1992), periphyton blooms have been noted more recently in clear-water lakes with high water quality (Vandeboncouer and others 2021). The conditions that led to the 2021 FAB in Crater Lake appeared to be associated with record high surface water temperature when the FAB initially formed in early summer, whereas the spatial distribution of the FAB was associated with the spatial distribution of crayfish. The overlap between the distribution of crayfish and FAB may seem surprising given that crayfish can readily graze on larger filamentous algae like Cladophora (Creed 1994), and crayfish grazing on Cladophora can even outweigh reduced grazing from snails (Nyström and others 1996; Dorn and Wojdak 2004). However, control of periphyton by grazers is most effective during the early, exponential growth phase of algae when grazers can consume new algal biomass almost as quickly as it is produced (Vandeboncouer and others 2021). Environmental factors that increase growth rates of algae (such as temperature) or reduce the consumption rate of grazers can allow algae to escape from grazer control during early successional stages (Vandeboncouer and others 2021). We suspect the lack of native zoobenthic grazers in crayfish dominated areas combined with extremely warm water temperature in early July 2021 allowed Cladophora to grow quickly and escape from grazer control in crayfish areas, thereby resulting in an overlap in crayfish and FAB spatial distribution.
With the exception of the 2021 FAB, nitrogen-fixing cyanobacteria (Calothrix sp.) and diatoms (Epithemia sp.) with endosymbiotic cyanobacteria dominated the periphyton community composition in both high and low crayfish areas of Crater Lake like other clear, low-nutrient lakes (Higgins and others 2003; Diehl and others 2018). While periphyton community composition was not statistically different at high and low crayfish density sites during summer 2020, sites with high crayfish population were the only locations where we observed Cladophora. Filamentous green algal cells are larger and have a higher nutrient demand, especially for nitrate and ammonium (Thybo-Christese and others 1993; John and Rindi 2015), compared with cyanobacteria and diatoms that they replace (Vadeboncoeur and others 2021). However, our nutrient limitation study showed that periphyton at high crayfish locations had a slightly higher N deficit compared to low crayfish areas. Also, as periphyton biomass accumulates, the redox gradients between the algae mat and the sediment may increase P flux from the substrate to the biofilm (Wood and others 2015; Vadeboncoeur and others 2021). Therefore, the presence of Cladophora may explain why nitrogen was the main nutrient limiting algae growth at high crayfish locations. However, Cladophora can also associate with nitrogen-fixing organisms (Dodds and Gudder 1992). Therefore, further research is needed to understand the differences in nutrient limitation for periphyton growth at sites with and without crayfish.
As mentioned above, it is unclear how much periphyton gets consumed by signal crayfish in Crater Lake, either algae within the biofilm or larger filamentous taxa like Cladophora, when available. It should be noted that macroinvertebrate biomass was low in crayfish dominated areas but were not completely absent. Thus, this high-quality food may still have been available for signal crayfish to eat and may have allowed crayfish to not feed heavily on algal food sources. Signal crayfish have been shown to prefer animal diets over plant diets more than other crayfish species (Guan and Wiles 1998; Larson and others 2017). Likewise, Guan and Wiles (1998) found that signal crayfish preferred macroinvertebrates more during spring and summer when their daily rations were higher. Our studies also corresponded to summer season.
While this study tests concepts associated with crayfish invasions (for example, trophic cascade, alterations to macroconsumer and autotrophic productivity, biomass, and diversity), we acknowledge the limitations of our work. We only investigated changes in the lake’s littoral habitat (up to 10 m depth), while the entire photic zone in Crater Lake regularly penetrates below 100 m. Likewise, we did not study how crayfish affect ecosystem rates (for example, N fixation, carbon sequestration, and cycling) and other important aspects of biofilm community composition. For example, biofilms are also comprised of bacteria, micro consumers (for example, benthic copepods, tardigrades), and protists, which significantly contribute to nutrient and carbon cycling. Biofilms can vary by depth and substrata type (for example, sediment, rocks). In addition, we did not study how crayfish induced ecosystem changes will affect the lake’s fisheries and higher trophic level food web. Therefore, additional resources are desperately needed to quantify the ecological conditions in the lake before the invasion of crayfish is completed in the remaining 20% of the lake shoreline.
Conclusion
We showed crayfish, through cascade effects, can increase overall nearshore productivity, favor the appearance of large filamentous algae, and modify algal nutrient limitation. Evidence suggests that the proliferation of crayfish within the last decade has led to dramatic ecological changes to the littoral environment of Crater Lake, suggesting further expansion may lead to denser and more widespread FABs as the climate warms. With changing climate, we have observed a change in invasion potential and nearshore expansion of an already well-established invasive species within a large mountain ecosystem.
Even in a lake already protected under the management of the USA National Park Service, the introduction and spread of crayfish had direct impacts on many levels of ecological organization, ultimately favoring a shoreline FAB in one of the clearest lakes in the world. Unfortunately, removal or control of crayfish once introduced into aquatic systems is extremely difficult using current technologies (Gherardi and others 2011; Manfrin and others 2019). Crayfish control or removal in Crater Lake is not presently feasible given the large size of the lake (35 km of shoreline), extensive rocky substrate, and movement of crayfish down to 200 m depth (Girdner and others 2018). Attempts at removing signal crayfish using extensive trapping in an area of Crater Lake over three years resulted in a reduction of larger size crayfish but a compensatory increase in total crayfish survival (data unpublished), similar to attempts at rusty crayfish removal at fish spawning sites in Lake Michigan (Kvistad and others, 2021). Emerging invasive control possibilities that include cutting edge genetic technologies (Simberloff 2021) give hope (and controversy) to future aquatic invasive species management options, although those technologies have yet to focus on crayfish.
Data Availability
Data and metadata are available at ‘‘Signal crayfish deteriorates littoral ecosystem, repository’’, Mendeley Data, V1, https://data.mendeley.com/datasets/vdprdysjbn/1.
References
Anderson MJ, Ellingsen KE, McArdle BH. 2006. Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693.
Bacon CR. 1983. Eruptive history of Mount Mazama and Crater Lake Caldera, cascade range, USA. J Volcanol Geotherm Res 18:57–115.
Bacon CR, Gardner JV, Mayer LA, Buktenica MW, Dartnell P, Ramsey DW, Robinson JE. 2002. Morphology, volcanism, and mass wasting in Crater Lake, Oregon. Bull Geol Soc Am 114:675–692.
Bernardo JM, Costa AM, Bruxelas S, Teixeira A. 2011. Dispersal and coexistence of two non-native crayfish species (Pacifastacus leniusculus and Procambarus clarkii) in NE Portugal over a 10-year period. Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2011047.
Bjurström L, Ruokonen T, Pursiainen M, Jones RI, Hämäläinen H. 2010. Impacts of the invasive signal crayfish on littoral macroinvertebrates of large boreal lakes: a pilot study in Lake Päijänne, Finland. Freshw Crayfish 17:177–182.
Brothers S, Vadeboncoeur Y, Sibley P. 2016. Benthic algae compensate for phytoplankton losses in large aquatic ecosystems. Glob Chang Biol 22:3865–3873. https://doi.org/10.1111/gcb.13306.
Buktenica MW, Girdner SF, Larson GL, McIntire CD. 2007. Variability of kokanee and rainbow trout food habits, distribution, and population dynamics, in an ultraoligotrophic lake with no manipulative management. Hydrobiologia 574:235–264. https://doi.org/10.1007/s10750-006-0355-1.
Cantonati M, Lowe RL. 2014. Lake benthic algae: toward an understanding of their ecology. Freshw Sci 33:475–486. https://doi.org/10.1086/676140.
Charlebois PM, Lamberti GA. 1996. Invading crayfish in a Michigan Stream: direct and indirect effects on periphyton and macroinvertebrates. J North Am Benthol Soc 15:551–563.
Charles DF, Knowles C, Davis RS. 2002. Protocols for the analysis of algal samples collected as part of the US Geological Survey National Water-Quality Assessment Program. Patrick Cent Environ Res Rep.
Creed RP. 1994. Direct and indirect effects of crayfish grazing in a stream community. Ecology 75:2091–2103. https://doi.org/10.2307/1941613.
Devlin SP, Vander Zanden MJ, Vadeboncoeur Y. 2016. Littoral–benthic primary production estimates: sensitivity to simplifications with respect to periphyton productivity and basin morphometry. Limnol Oceanogr Methods 14:138–149.
Diehl S, Thomsson G, Kahlert M, Guo J, Karlsson J, Liess A. 2018. Inverse relationship of epilithic algae and pelagic phosphorus in unproductive lakes: roles of N2 fixers and light. Freshw Biol 63:662–675. https://doi.org/10.1111/fwb.13103.
Dodds WK. 1991. Community interactions between the filamentous alga Cladophora glomerata (L.) Kuetzing, its epiphytes, and epiphyte grazers. Oecologia 85:572–580.
Dodds WK, Gudder DA. 1992. The ecology of Cladophora. J Phycol 28:415–427. https://doi.org/10.1111/j.0022-3646.1992.00415.x.
Dorn N, Wojdak J. 2004. The role of omnivorous crayfish in littoral communities. Oecologia 140:150–159.
Ercoli F, Ruokonen TJ, Koistinen S, Jones RI, Hämäläinen H. 2015. The introduced signal crayfish and native noble crayfish have different effects on sublittoral macroinvertebrate assemblages in boreal lakes. Freshw Biol 60:1688–1698. https://doi.org/10.1111/fwb.12601.
Fitzsimons JD, Jonas JL, Claramunt RM, Williston B, Williston G, Marsden JE, Ellrott BJ, Honeyfield DC. 2007. Influence of egg predation and physical disturbance on lake trout Salvelinus namaycush egg mortality and implications for life-history theory. J Fish Biol 71:1–16. https://doi.org/10.1111/j.1095-8649.2007.01437.x.
Gherardi F, Aquiloni L, Diéguez-Uribeondo J, Tricarico E. 2011. Managing invasive crayfish: is there a hope? Aquat Sci 73:185–200. https://doi.org/10.1007/s00027-011-0181-z.
Girdner SF, Ray AM, Buktenica MW, Hering DK, Mack JA, Umek JW. 2018. Replacement of a unique population of newts (Taricha granulosa mazamae) by introduced signal crayfish (Pacifastacus leniusculus) in Crater Lake, Oregon. Biol Invasions 20:721–740.
Girdner S, Mack J, Buktenica M. 2020. Impact of nutrients on photoacclimation of phytoplankton in an oligotrophic lake measured with long-term and high-frequency data: implications for chlorophyll as an estimate of phytoplankton biomass. Hydrobiologia 847:1817–1830. https://doi.org/10.1007/s10750-020-04213-1.
Guan RZ, Wiles PR. 1998. Feeding ecology of the signal crayfish Pacifastacus leniusculus in a British lowland river. Aquaculture 169:177–193. https://doi.org/10.1016/S0044-8486(98)00377-9.
Hansen GJA, Hein CL, Roth BM, Vander Zanden MJ, Gaeta JW, Latzka AW, Carpenter SR. 2013. Food web consequences of long-term invasive crayfish control. Can J Fish Aquat Sci 70:1109–1122. https://doi.org/10.1139/cjfas-2012-0460.
Hargreaves BR, Girdner SF, Buktenica MW, Collier RW, Urbach E, Larson GL. 2007. Ultraviolet radiation and bio-optics in Crater Lake, Oregon. Hydrobiologia 574:107–140. https://doi.org/10.1007/s10750-006-0348-0.
Higgins SN, Kling HJ, Hecky RE, Taylor WD, Bootsma HA. 2003. The community composition, distribution, and nutrient status of epilithic periphyton at five rocky littoral zone sites in Lake Malawi, Africa. J Great Lakes Res 29:181–189.
Jackson MC, Jones T, Milligan M, Sheath D, Taylor J, Ellis A, England J, Grey J. 2014. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshw Biol 59:1123–1135. https://doi.org/10.1111/fwb.12333.
John DM, Rindi F. 2015. Chapter 8—filamentous (nonconjugating) and plantlike green algae. In: Wehr JD, Sheath RG, Kociolek JP, Eds. Freshwater algae of North America. Aquatic ecology, 2nd edn. Boston: Academic Press. pp 375–427.
Kvistad JT, Buckley JT, Robinson KM, Galarowicz TL, Claramunt RM, Clapp DF, O’Neill P, Chadderton WL, Tucker AJ, Herbert M. 2021. Size segregation and seasonal patterns in rusty crayfish Faxonius rusticus distribution and abundance on northern Lake Michigan spawning reefs. J Great Lakes Res 47(4):1050–1064. https://doi.org/10.1016/j.jglr.2021.03.009.
Larson GL, Collier R, Buktenica M. 2007. Long-term limnological research and monitoring at Crater Lake, Oregon. Hydrobiologia 574:1–11. https://doi.org/10.1007/s10750-006-0342-6.
Larson ER, Abbott CL, Usio N, Azuma N, Wood KA, Herborg LM, Olden JD. 2012. The signal crayfish is not a single species: cryptic diversity and invasions in the Pacific Northwest range of Pacifastacus leniusculus. Freshw Biol 57:1823–1838. https://doi.org/10.1899/12-051.1.
Larson ER, Twardochleb LA, Olden JD. 2017. Comparison of trophic function between the globally invasive crayfishes Pacifastacus leniusculus and Procambarus clarkii. Limnology 18:275–286. https://doi.org/10.1007/s10201-016-0505-8.
Lenth RV. 2021. emmeans: estimated marginal means, aka least-squares means.
Lodge DM, Kershner MW, Aloi JE, Covich AP. 1994. Effects of an omnivorous crayfish (Orconectes rusticus) on a freshwater littoral food web. Ecology 75:1265–1281. https://doi.org/10.2307/1937452.
Lodge DM, Taylor CA, Holdich DM, Skurdal J. 2000. Nonindigenous crayfishes threaten North American freshwater biodiversity: lessons from Europe. Fisheries 25:7–20.
Lodge DM, Deines A, Gherardi F, Yeo DCJ, Arcella T, Baldridge AK, Barnes MA, Chadderton WL, Feder JL, Gantz CA, Howard GW, Jerde CL, Peters BW, Peters JA, Sargent LW, Turner CR, Wittmann ME, Zeng Y. 2012. Global Introductions of Crayfishes: evaluating the impact of species invasions on ecosystem services. Annu Rev Ecol Evol Syst 43:449–472. https://doi.org/10.1146/annurev-ecolsys-111511-103919.
Lottig NR, Phillips JS, Batt RD, Scordo F, Williamson TJ, Carpenter SR, Chandra S, Hanson PC, Solomon CT, Vanni MJ, Zwart J. 2022. Estimating pelagic primary production in lakes: comparison of 14C incubation and free-water O2 approaches. Limnol Oceanogr Methods 20:34–45. https://doi.org/10.1002/lom3.10471.
Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world’s worst invasive alien species. A selection from the Global Invasive Species Database. Aliens 12:1–12.
Ludlam JP, Banks BT, Magoulick DD. 2015. Density-dependent effects of omnivorous stream crayfish on benthic trophic dynamics. Freshw Crayfish 21(1):165–170. https://doi.org/10.5869/fc.2015.v21-1.165.
Luttenton MR, Lodge DM, Horgan MJ. 1998. Effects of three orconectes crayfishes on epilithic microalgae: a laboratory experiment. Crustaceana 71(8):845–855. https://doi.org/10.1163/156854098X00860.
Maberly SC, King L, Dent MM, Jones RI, Gibson CE. 2002. Nutrient limitation of phytoplankton and periphyton growth in upland lakes. Freshw Biol 47:2136–2152. https://doi.org/10.1046/j.1365-2427.2002.00962.x.
Manfrin C, Souty-Grosset C, Anastácio PM, Reynolds J, Giulianini PG. 2019. Detection and control of invasive freshwater crayfish: from traditional to innovative methods. Diversity 11(1):5. https://doi.org/10.3390/d11010005.
Marker AFH. 1972. The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin. Freshw Biol Biol 2:361–385.
McCarthy JM, Hein CL, Olden JD, Vander Zanden MJ. 2006. Coupling long-term studies with meta-analysis to investigate impacts of non-native crayfish on zoobenthic communities. Freshw Biol 51:224–235. https://doi.org/10.1111/j.1365-2427.2005.01485.x.
McIntire CD, Larson GL, Truitt RE. 2007. Seasonal and interannual variability in the taxonomic composition and production dynamics of phytoplankton assemblages in Crater Lake, Oregon. Hydrobiologia 574:179–204. https://doi.org/10.1007/s10750-006-0352-4.
Messager ML, Olden JD. 2018. Individual-based models forecast the spread and inform the management of an emerging riverine invader. Divers Distrib 24:1816–1829. https://doi.org/10.1111/ddi.12829.
Momot WT. 1984. Crayfish production: a reflection of community energetics. J Crustac Biol 4(1):35–54. https://doi.org/10.2307/1547894.
Nyström P, Brönmark C, Granéli W. 1996. Patterns in benthic food webs: a role for omnivorous crayfish? Freshw Biol 36:631–646. https://doi.org/10.1046/j.1365-2427.1996.d01-528.x.
Nyström P, Bronmark C, Graneli W. 1999. Influence of an exotic and a native crayfish species on a littoral benthic community. Oikos 85:545–553.
Nyström P, Svensson O, Lardner B, Brönmark C, Granéli W. 2001. The influence of multiple introduced predators on a littoral pond community. Ecology 82:1023–1039. https://doi.org/10.1890/0012-9658(2001)082[1023:TIOMIP]2.0.CO.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2019. vegan: Community Ecology Package. R package version 2.5-6. https://cran.r-project.org/package=vegan.
Ooue K, Ishiyama N, Ichimura M, Nakamura F. 2019. Environmental factors affecting the invasion success and morphological responses of a globally introduced crayfish in floodplain waterbodies. Biol Invasions 21:2639–2652. https://doi.org/10.1007/s10530-019-02008-7.
Peay S, Guthrie N, Spees J, Nilsson E, Bradley P. 2009. The impact of signal crayfish (Pacifastacus leniusculus) on the recruitment of salmonid fish in a headwater stream in Yorkshire, England. Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2010003.
Peters JA, Lodge DM. 2009. Littoral zone. In: Likens GE, Ed. Encyclopedia of inland waters, . Oxford: Academic Press. pp 79–87.
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team. RC. 2020. nlme: linear and nonlinear mixed effects models. https://cran.r-project.org/package=nlme.
R Core Team. 2020. R: a language and environment for statistical computing. https://www.r-project.org/.
Ruokonen TJ, Karjalainen J, Hämäläinen H. 2014. Effects of an invasive crayfish on the littoral macroinvertebrates of large boreal lakes are habitat specific. Freshw Biol 59:12–25. https://doi.org/10.1111/fwb.12242.
Ruokonen TJ, Ercoli F, Hämäläinen H. 2016. Are the effects of an invasive crayfish on lake littoral macroinvertebrate communities consistent over time? Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2016018.
Sadro S, Nelson CE, Melacka JM. 2011. Linking diel patterns in community respiration to bacterioplankton in an oligotrophic high-elevation lake. Limnol Oceanogr 56:540–550.
Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089.
Scordo F, Lottig NR, Fiorenza JE, Culpepper J, Simmons J, Seitz C, Krynak EM, Suenaga E, Chandra S. 2022a. Hydroclimate variability affects habitat-specific (open water and littoral) lake metabolism. Water Resour Res. https://doi.org/10.1029/2021WR031094.
Scordo F, Girdner SF, Chandra S. 2022b. Signal crayfish deteriorates littoral ecosystem, repository. Mendeley Data, V1, https://data.mendeley.com/datasets/vdprdysjbn/1.
Simberloff D. 2021. Maintenance management and eradication of established aquatic invaders. Hydrobiologia 848:2399–2420. https://doi.org/10.1007/s10750-020-04352-5.
Stenroth P, Nyström P. 2003. Exotic crayfish in a brown water stream: effects on juvenile trout, invertebrates and algae. Freshw Biol 48:466–475. https://doi.org/10.1046/j.1365-2427.2003.01020.x.
Spear MJ, Walsh JR, Ricciardi A, Zanden MJV. 2021. The invasion ecology of sleeper populations: prevalence, persistence, and abrupt shifts. BioScience 71(4):357–369. https://doi.org/10.1093/biosci/biaa168.
Stetler JT, Girdner S, Mack J, Winslow LA, Leach TH, Rose KC. 2021. Atmospheric stilling and warming air temperatures drive long-term changes in lake stratification in a large oligotrophic lake. Limnol Oceanogr 66:954–964. https://doi.org/10.1002/lno.11654.
Tank JL, Reisinger AJ, Rosi EJ. 2017. Chapter 31—Nutrient limitation and uptake. In: Lamberti GA, Hauer FR, Eds. Methods in stream ecology, 3rd edn. Oxford: Academic Press. pp 147–171.
Thybo-Christesen M, Rasmussen MB, Blackburn TH. 1993. Nutrient fluxes and growth of Cladophora sericea in a shallow Danish bay. Mar Ecol Prog Ser 100:273–281.
Tromboni F, Dodds WK, Neres-Lima V, Zandonà E, Moulton TP. 2017. Heterogeneity and scaling of photosynthesis, respiration, and nitrogen uptake in three Atlantic Rainforest streams. Ecosphere 8:e01959. https://doi.org/10.1002/ecs2.1959.
Twardochleb LA, Olden JD, Larson ER. 2013. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci 32:1367–1382. https://doi.org/10.1899/12-203.1.
Usio N. 2000. Effects of crayfish on leaf processing and invertebrate colonisation of leaves in a headwater stream: decoupling of a trophic cascade. Oecologia 124:608–614. https://doi.org/10.1007/s004420000422.
Usio N, Imada M, Akasaka M, Taka N. 2009. Effects of pond management on the distributions of aquatic invaders in Japanese farm ponds. feature ‘biological invasions and human activities.’ Jpn J Limnol 70:261–266.
Vadeboncoeur Y, Steinman AD. 2002. Periphyton function in lake ecosystems. Sci World J 2:1449–1468.
Vadeboncoeur Y, Jeppesen E, Vander Zanden MJ, Schierup HH, Christoffersen K, Lodge DM. 2003. From Greenland to green lakes: cultural eutrophication and the loss of benthic pathways in lakes. Limnol Oceanogr 48:1408–1418.
Vadeboncoeur Y, Devlin SP, McIntyre PB, Vander Zanden MJ. 2014. Is there light after depth? Distribution of periphyton chlorophyll and productivity in lake littoral zones. Freshw Sci 33:524–536.
Vadeboncoeur Y, Moore MV, Stewart SD, Chandra S, Atkins KS, Baron JS, Bouma-Gregson K, Brothers S, Francoeur SN, Genzoli L, Higgins SN, Hilt S, Katona LR, Kelly D, Oleksy IA, Ozersky T, Power ME, Roberts D, Smits AP, Timoshkin O, Tromboni F, Vander Zanden MJ, Volkova EA, Waters S, Wood SA, Yamamuro M. 2021. Blue waters, green bottoms: benthic filamentous algal blooms are an emerging threat to clear lakes worldwide. Bioscience 71:1011–1027.
van der Wal J, Dorenbosch M, Immers A, Vidal Forteza C, Geurts J, Peeters E, Koese B, Bakker E. 2013. Invasive crayfish threaten the development of submerged macrophytes in lake restoration. PLoS ONE 8:e78579.
Weber LM, Lodge DM. 1990. Periphytic food and predatory crayfish: relative roles in determining snail distribution. Oecologia 82:33–39. https://doi.org/10.1007/BF00318530.
Whitmore N. 1997. Population ecology of the freshwater crayfish Paranephrops zealandicus and its effect on the community structure of a lowland bush stream.
Wood SA, Depree C, Brown L, McAllister T, Hawes I. 2015. Entrapped sediments as a source of phosphorus in epilithic cyanobacterial proliferations in low nutrient rivers. PLoS ONE 10:e0141063.
Vander Zanden MJ, Chandra S, Park SK, Vadeboncoeur Y, Goldman CR. 2006. Efficiencies of benthic and pelagic trophic pathways in a subalpine lake. Can J Fish Aquat Sci 63:2608–2620.
Vander Zanden MJ, Vadeboncoeur Y, Chandra S. 2011. Fish reliance on littoral–benthic resources and the distribution of primary production in lakes. Ecosystems 14:894–903.
Vander Zanden MJ, Vadeboncoeur Y. 2020. Putting the lake back together 20 years later: what in the benthos have we learned about habitat linkages in lakes? Inl Waters 10:305–321. https://doi.org/10.1080/20442041.2020.1712953.
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer.
Acknowledgements
Funding was provided to UNR by the National Park Service (cooperative agreement CESU-2021-001030). The authors thank the University of Nevada’s College and University’s Global Water Center. Some of the concepts in this manuscript were developed as part of National Science Foundation support through Division of Environmental Biology award 1939502 to SC and Yvonne Vadeboncoeur.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions: FS performed data analysis and led manuscript writing. SFG and SC assisted with the original idea, and the experiment design. SFG lead the lake monitoring program. SFG, CS, and ASP, helped process the data. All authors contributed to performing the experiments, conceptual model development, the literature review, and the writing of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scordo, F., Girdner, S.F., San Pedro, A. et al. Deterioration of the Littoral–Benthic Ecosystem Following Recent Expansion of Signal Crayfish (Pacifastacus leniusculus) in the World’s Clearest Large Lake. Ecosystems 26, 1489–1503 (2023). https://doi.org/10.1007/s10021-023-00846-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00846-0