Abstract
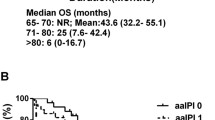
Clinicopathological risk factors for a poor prognosis were investigated in elderly patients with malignant lymphoma of the central nervous system. A total of 82 pathologically confirmed, CD20-positive, diffuse large B-cell lymphoma patients aged 71 years or older who underwent therapeutic intervention in the Tohoku and Niigata area in Japan were retrospectively reviewed. A univariate analysis was performed by the log-rank test using the Kaplan–Meier method. A Cox proportional hazards model was used for multivariate analysis of risk factors. Of the 82 patients, 39 were male and 43 were female, and their median age at onset was 75 years. At the end of the study, there were 34 relapse-free patients (41.5%), 48 relapse cases (58.5%), median progression-free survival was 18 months, and median overall survival (OS) was 26 months; there were 41 deaths and 41 survivors. Multivariate analysis of median OS showed that Karnofsky Performance Status less than 60% 3 months after treatment (p = 0.022, hazard ratio (HR) = 2.591) was the clinical risk factor, and double expressor lymphoma (p = 0.004, HR = 3.163), expression of programmed death-ligand 1 in tumor infiltrating lymphocytes or tumor-associated macrophages (p < 0.001, HR = 5.455), and Epstein–Barr virus infection (p = 0.031, HR = 5.304) were the pathological risk factors.



Similar content being viewed by others
References
Corn BW, Marcus SM, Topham A et al (1997) Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer 79:2409–2413
Daras M, DeAngelis LM (2013) Management of elderly patients with primary central nervous system lymphoma. Curr Neurol Neurosci Rep 13:344
Ferreri AJ, Blay JY, Reni M et al (2003) Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol 21:266–272
Abrey LE, Ben-Porat L, Panageas KS et al (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol 24:5711–5715
Tateishi K, Miyake Y, Nakamura T et al (2021) Primary central nervous system lymphoma: clinicopathological and genomic insights for therapeutic development. Brain Tumor Pathol 38:173–182
Camilleri-Broet S, Criniere E, Broet P et al (2006) A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood 107:190–196
Niparuck P, Boonsakan P, Sutthippingkiat T et al (2019) Treatment outcome and prognostic factors in PCNSL. Diagn Pathol 14:56
Marcus C, Maragkos GA, Alterman RL et al (2021) GCB-type is a favorable prognostic factor in primary CNS diffuse large B-cell lymphomas. J Clin Neurosci 83:49–55
Hatzl S, Posch F, Deutsch A et al (2020) Immunohistochemistry for c-myc and bcl-2 overexpression improves risk stratification in primary central nervous system lymphoma. Hematol Oncol 38:277–283
Furuse M, Kuwabara H, Ikeda N et al (2020) PD-L1 and PD-L2 expression in the tumor microenvironment including peritumoral tissue in primary central nervous system lymphoma. BMC Cancer 20:277
Cho H, Kim SH, Kim SJ et al (2017) Programmed cell death 1 expression is associated with inferior survival in patients with primary central nervous system lymphoma. Oncotarget 8:87317–87328
Hayano A, Komohara Y, Takashima Y et al (2017) Programmed cell death ligand 1 expression in primary central nervous system lymphomas: a clinicopathological study. Anticancer Res 37:5655–5666
Cho I, Lee H, Yoon SE et al (2020) Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer 20:120
Takashima Y, Kawaguchi A, Sato R et al (2019) Differential expression of individual transcript variants of PD-1 and PD-L2 genes on Th-1/Th-2 status is guaranteed for prognosis prediction in PCNSL. Sci Rep 9:10004
Berghoff AS, Ricken G, Widhalm G et al (2014) PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin Neuropathol 33:42–49
Sasayama T, Tanaka K, Mizowaki T et al (2016) Tumor-associated macrophages associate with cerebrospinal fluid interleukin-10 and survival in primary central nervous system lymphoma (PCNSL). Brain Pathol 26:479–487
Komohara Y, Horlad H, Ohnishi K et al (2011) M2 macrophage/microglial cells induce activation of Stat3 in primary central nervous system lymphoma. J Clin Exp Hematop 51:93–99
Marcelis L, Antoranz A, Delsupehe AM et al (2020) In-depth characterization of the tumor microenvironment in central nervous system lymphoma reveals implications for immune-checkpoint therapy. Cancer Immunol Immunother 69:1751–1766
Jamal SE, Li S, Bajaj R et al (2014) Primary central nervous system Epstein–Barr virus-positive diffuse large B-cell lymphoma of the elderly: a clinicopathologic study of five cases. Brain Tumor Pathol 31:265–273
Sugita Y, Terasaki M, Niino D et al (2010) Epstein–Barr virus-associated primary central nervous system lymphomas in immunocompetent elderly patients: analysis for latent membrane protein-1 oncogene deletion and EBNA-2 strain typing. J Neurooncol 100:271–279
Kitai R, Matsuda K, Adachi E et al (2010) Epstein–Barr virus-associated primary central nervous system lymphoma in the Japanese population. Neurol Med Chir (Tokyo) 50:114–118
Asano K, Yamashita Y, Ono T et al (2021) The real-world status and risk factors for a poor prognosis in elderly patients with primary central nervous system malignant lymphomas: a multicenter, retrospective cohort study of the Tohoku Brain Tumor Study Group. Int J Clin Oncol 27:77–94
Topalian SL, Taube JM, Anders RA et al (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287
Chow LQM, Haddad R, Gupta S et al (2016) Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 34:3838–3845
Green MR, Monti S, Rodig SJ et al (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116:3268–3277
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Green TM, Young KH, Visco C et al (2012) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 30:3460–3467
Matsubara T, Takada K, Azuma K et al (2019) A Clinicopathological and prognostic analysis of PD-L2 expression in surgically resected primary lung squamous cell carcinoma. Ann Surg Oncol 26:1925–1933
Tomita S, Kikuti YY, Carreras J et al (2015) Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Mod Pathol 28:1286–1296
Hu S, Xu-Monette ZY, Tzankov A et al (2013) MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 121:4021–4031 (quiz 4250)
Abrey LE, Batchelor TT, Ferreri AJ et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034–5043
Wang CC, Carnevale J, Rubenstein JL (2014) Progress in central nervous system lymphomas. Br J Haematol 166:311–325
Kluin PMDM, Ferry JA (2008) Primary diffuse large B-cell lymphoma of the CNS. IARC Press, Lyon
Shiozawa E, Yamochi-Onizuka T, Takimoto M et al (2007) The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leuk Res 31:1579–1583
Phang KC, Hussin NH, Abdul RF et al (2019) Characterisation of immunogenotypes of diffuse large B-cell lymphoma. Malays J Pathol 41:101–124
Umemura H, Homma M, Shiozwa E et al (2012) Immunohistochemical analysis of the cell cycle-associated proteins in diffuse large B-cell lymphoma. Showa IKaishi 72:108–117
Seki R, Ohshima K, Fujisaki T et al (2009) Prognostic impact of immunohistochemical biomarkers in diffuse large B-cell lymphoma in the rituximab era. Cancer Sci 100:1842–1847
Radotra BD, Parkhi M, Chatterjee D et al (2020) Clinicopathological features of primary central nervous system diffuse large B cell lymphoma: experience from a Tertiary Center in North India. Surg Neurol Int 11:424
Aukema SM, Siebert R, Schuuring E et al (2011) Double-hit B-cell lymphomas. Blood 117:2319–2331
Riedell PA, Smith SM (2018) Double hit and double expressors in lymphoma: definition and treatment. Cancer 124:4622–4632
Ma Z, Niu J, Cao Y et al (2020) Clinical significance of “double-hit” and “double-expression” lymphomas. J Clin Pathol 73:126–138
Xu-Monette ZY, Xiao M, Au Q et al (2019) Immune profiling and quantitative analysis decipher the clinical role of immune-checkpoint expression in the tumor immune microenvironment of DLBCL. Cancer Immunol Res 7:644–657
Godfrey J, Tumuluru S, Bao R et al (2019) PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood 133:2279–2290
Wu P, Wu D, Li L et al (2015) PD-L1 and survival in solid tumors: a meta-analysis. PLoS ONE 10:e0131403
Zhao T, Li C, Wu Y et al (2017) Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: a meta-analysis. PLoS ONE 12:e0176822
Marchesi F, Cirillo M, Bianchi A et al (2015) High density of CD68+/CD163+ tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematol Oncol 33:110–112
Kamada T, Togashi Y, Tay C et al (2019) PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci USA 116:9999–10008
Kumagai S, Togashi Y, Kamada T et al (2020) The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 21:1346–1358
Oyama T, Ichimura K, Suzuki R et al (2003) Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol 27:16–26
Gulley ML (2001) Molecular diagnosis of Epstein–Barr virus-related diseases. J Mol Diagn 3:1–10
Acknowledgements
The authors would like to express their appreciation to all those who contributed to our study. Yuichi Sato MD, PhD (Department of Neurosurgery, Iwate Medical University), Professor Masazumi Fujii MD, PhD (Department of Neurosurgery, Fukushima Medical University), Yoshihiro Kameoka MD, PhD, (Department of Hematology, Nephrology and Rheumatology, Akita University Graduate School of Medicine), all the doctors at all the institutions and the members of the Tohoku Brain Tumor Study Group, and all the staff of the Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
KA and HO contributed to the concept and design of the study. KA and MM contributed to the acquisition and analysis of the data. AK and KA contributed to central pathological diagnosis. All authors contributed to drafting the text and preparing the figure. KA, YY, TO, MN, TB, KM, MI, MK, and TF contributed to acquisition of the data in individual institutions. KS, YS, KO, YF, HS, HO, and TT contributed to supervision in individual institutions. YS, CK, TK, and TT contributed to supervision and advising on the whole project.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10014_2022_427_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1 Kaplan–Meier curves of double hit score (DHS) 0, 1, and 2. OS comparing DHS 0 (light gray line), DHS 1 (gray line), and DHS 2 (black line). There are significant differences between DHS 0 vs DHS 2 and DHS 1 vs DHS 2 (p=0.035 and p=0.017, respectively) (TIF 41239 KB)
Rights and permissions
About this article
Cite this article
Asano, K., Yamashita, Y., Ono, T. et al. Clinicopathological risk factors for a poor prognosis of primary central nervous system lymphoma in elderly patients in the Tohoku and Niigata area: a multicenter, retrospective, cohort study of the Tohoku Brain Tumor Study Group. Brain Tumor Pathol 39, 139–150 (2022). https://doi.org/10.1007/s10014-022-00427-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-022-00427-4