Abstract
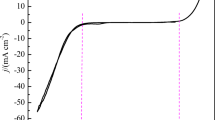
The mechanism and kinetics of zinc electrochemical nucleation and growth onto glassy carbon surface, from Zn(II) ions dissolved in the choline chloride (ChCl)–urea eutectic mixture, reline, at different temperatures, T, within the 303 to 363 K range, are reported for the first time. From the potentiodynamic study, the exchange current density, j0, and the energy transfer coefficient, α, of the Zn(II)DES + 2e−GCE/Zn(s) ↔ Zn(s) reaction were estimated as a function of T. It was found that while j0 depends exponentially on T, α= 0.12 ± 0.02 remains almost constant. Furthermore, the activation energy, E* = (33 ± 0.2) kJmol−1, of this reaction was assessed from the Arrhenius-type plot (ln j0 vs. T−1). Analysis of the potentiostatic current density transients allowed to establish that the zinc electrodeposition mechanism occurs via the simultaneous presence of a Langmuir-type adsorption–desorption equilibrium, an instantaneous nucleation process with two-dimensional (2D) growth limited by the rate of lattice incorporation, and a diffusion-controlled three-dimensional nucleation and growth contribution (3D), which is particularly notorious at 348 K. This is the first time that a 2D-3D nucleation transition has been observed during the electrochemical deposition of metals from deep eutectic solvents (DES). From the 2D nucleation process contribution to the total current density, it was possible to determine the surface roughness factor and the electrochemically active surface area of the glassy carbon electrode (GCE). The time evolution of the Zn monolayer formation onto the GCE was also reported.








Similar content being viewed by others
References
Prieto JE, Markov I (2017) Stranski-Krastanov mechanism of growth and the effect of misfit sign on quantum dots nucleation. Surf Sci 664:172–184
Philipp R, Retter U (1995) On transition from 2D to 3D nucleation in the anodic film formation of thiourea at the mercury/electrolyte interphase. Electrochima Acta 40:1581–1585
Palomar-Pardavé M, González I, Batina N (2000) New insights into evaluation of kinetic parameters for potentiostatic metal deposition with underpotential and overpotential deposition processes. J Phys Chem B 104:3545–3555
Romero-Romo M, Aldana-González J, Botello LE, Montes de Oca MG, Ramírez-Silva MT, Corona-Avendaño S, Palomar-Pardavé M (2017) Electrochemical nucleation and growth of Cu onto Au nanoparticles supported on a Si (111) wafer electrode. J Electroanal Chem 791:1–7
Gupta A, Srivastava C (2021) Assessment of the nucleation and growth mechanism of copper electrodeposition over graphene oxide. Metall and Mater Trans A 52A:2522–2533
Rezaei M, Hadi Tabaian S, Fatmehsari Haghshenas D (2012) Nucleation and growth of Pd nanoparticles during electrocrystallization on pencil graphite. Electrochimica Acta 59:360– 366
Danaee I (2013) 2D–3D nucleation and growth of palladium on graphite electrode. J Ind Eng Chem 19:1008–1013
Arbib M, Zhang B, Lazarov V, Stoychev D, Milchev A, Buess-Herman C (2001) Electrochemical nucleation and growth of rhodium on gold substrates. J Electroanal Chem 510:67–77
Schulz EN, Salinas DR, García SG (2010) Electrodeposition of rhodium onto a pre-treated glassy carbon surface. Eletrochemistry Communications 12:583–586
Schulz EN, Ruderman A, Soldano GJ, García SG, Santos E (2015) Key role of anions in the 2D–3D electrochemical deposition of Rh on Ag electrodes. Electrochim Acta 178:813–822
Zhao C, Qiu C, Deng S, Sun X, Gao Y, Cao Y, Zhuo H, Zhuang G, Zhong X, Wei Z, Yao Z, Wang J-G (2019) 2D–3D transformation of palladium and gold nanoparticles on functionalized Mo2C by multiscale simulation. Appl Surf Sci 481:554–563
Chmielewski M, Grzeszczuk M, Kalenik J, Kepas-Suwara A (2010) Evaluation of the potential dependence of 2D–3D growth rates and structures of polypyrrole films in aqueous solutions of hexafluorates. J Electroanal Chem 647:169–180
Li X, Cao Y, Yang G (2010) Thermodynamic theory of two-dimensional to three-dimensional growth transition in quantum dots self-assembly. Phys Chem Chem Phys 12:4768–4772
Trejo G, Ortega B, Meas Y, Ozil P, Chainet E, Nguyen B (1998) Nucleation and growth of zinc from chloride concentrated solutions. J Electrochem Soc 145:4090–4097
Trejo G, Ruiz H, Ortega Borges R, Meas Y (2001) Influence of polyethoxylated additives on zinc electrodeposition from acid solutions. J Appl Electrochem 31:685–692
Ballesteros JC, Díaz-Arista P, Meas Y, Ortega R, Trejo G (2007) Zinc electrodeposition in the presence of polyethylene glycol 20000. Electrochim Acta 52:3686–3696
Rojas-Hernández A, Ramírez MT, González I, Ibañez JG (1995) Predominace-zone diagrams in solution chemistry. J Chem Educ 72:1099–1105
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082
Aldana-González J, Sampayo-Garrido A, Montes de Oca-Yemha MG, Sánchez W, Ramírez-Silva MT, Arce-Estrada EM, Romero-Romo M, Palomar-Pardavé M (2019) Electrochemical nucleation and growth of Mn and Mn-Zn alloy from leached liquors of spent alkaline batteries using a deep eutectic solvent. J Electrochem Soc 166:D199–D204
Xie X, Zou X, Lu X, Lu C, Cheng H, Xu Q, Zhou Z (2016) Electrodeposition of Zn and Cu–Zn alloy from ZnO/CuO precursors in deep eutectic solvent. Appl Surf Sci 385:481–489
Pitner WR, Hussey CL (1997) Electrodeposition of zinc from the Lewis acidicaluminum chloride-1-methyl-3-ethylimidazolium chloride room temperature molten salt. J Electrochem Soc 144:3095–3103
Pereira NM, Pereira CM, Araújo JP, Silva AF (2017) Zinc electrodeposition from deep eutectic solvent containing organic additives. J Electroanal Chem 801:545–551
Alesary HF, Cihangir S, Ballantyne AD, Harris RC, Weston DP, Abbott AP, Ryder KS (2019) Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim Acta 304:118–130
Pölzler M, Whitehead AH, Gollas B (2010) A study of zinc electrodeposition from zinc chloride: choline chloride: ethylene glycol. ECS Trans 25:43–55
Bao Q, Zhao L, Jing H, Mao A (2018) Electrodeposition of zinc from low transition temperature mixture formed by choline chloride + lactic acid. Materials Today Communications 14:249–253
Abbott AP, Barron JC, Frisch G, Ryder KS, Silva AF (2011) The effect of additives on zinc electrodeposition from deep eutectic solvents. Electrochim Acta 56:5272–5279
Pölzler M, Whitehead AH, Gollas B (2010) A study of zinc electrodeposition from zinc chloride: choline chloride: ethylene glycol. Physical & Analytical Electrochemistry in Ionic Liquids 25:43–55
Aldana-González J, Romero-Romo M, Robles-Peralta J, Morales-Gil P, Palacios-González E, Ramírez-Silva MT, Mostany J, Palomar-Pardavé M (2018) On the electrochemical formation of nickel nanoparticles onto glassy carbon from a deep eutectic solvent. Electrochim Acta 276:417–423
Palomar-Pardavé M, Aldana-González J, Botello LE, Arce-Estrada EM, Ramírez-Silva MT, Mostany J, Romero-Romo M (2017) Influence of temperature on the thermodynamics and kinetics of cobalt electrochemical nucleation and growth. Electrochim Acta 241:162–169
Palomar-Pardavé M, Miranda-Hernández M, González I, Batina N (1998) Detailed characterization of potentiostatic current transients with 2D–2D and 2D–3D nucleation transitions Surf. Sci 399:80–95
Scharifker BR, Mostany J (1984) Three-dimensional nucleation with diffusion controlled growth. Part I: Number density of active sites and nucleation rates per site. J Electroanal Chem 177:13–23
Ustarroz J (2020) Current atomic-level understanding of electrochemical nucleation and growth on low-energy surfaces. Curr Opin Electrochem 19:144–152
Alonzo DC, Scharifker BR (1989) On the underpotential-overpotential transition in the deposition of silver on platinum. J Electroanal Chem 274:167–178
Milchev A, Krastev I (2011) Two-dimensional progressive and instantaneous nucleation with overlap: the case of multi-step electrochemical reactions. Electrochim Acta 56:2399–2403
Kim J, Jerkiewicz G (2017) Influence of the surface roughness of platinum electrodes on the calibration of the electrochemical quartz-crystal nanobalance. Anal Chem 89:7462–7469
Aldana-González J, Olvera-García J, Montes de Oca MG, Romero-Romo M, Ramírez-Silva MT, Palomar-Pardavé M (2015) Electrochemical quantification of the electro-active surface area of Au nanoparticles supported onto an ITO electrode by means of Cu upd. Electrochem Commun 56:70–74
Frumkin F (1963) in Advances in electrochemistry and electrochemical engineering, Vol. 3 Delahay P, Tobias CW Editors 163 Interscience New York
Trasatti S, Petri 0A (1991) Real surface area measurements in electrochemistry, Pure Appl Chem 63:771–734
Zheng G, Popov BN, White RE (1994) Use of underpotential deposition of zinc to mitigate hydrogen absorption into Monel K500. J Electrochem Soc 141:1220–1224
Serruya A, Mostany J, Scharifker BR (1993) Spatial distributions and saturation number densities of lead nuclei deposited on vitreous carbon electrodes. J Chem Soc Faraday Trans 89:255−261
Acknowledgements
The authors would like to thank CONACyT for project 258487. GVG thanks CONACyT for the scholarship granted to pursue postgraduate studies. JAG, MRR, MTRS, MMMTL, and MPP wish to thank the SNI for the distinction of their membership. The authors are indebted to Prof. DSc Vessela Tsakova for the invitation to submit a contribution to this memorial issue of JSSE. We would like to thank Dra. Perla Morales-Gil (IMP) and Dra. Elsa Miriam Arce-Estrada (IPN) for XPS and SEM-EDX facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was dedicated to the memory of Prof. Alexander Milchev, one of the most outstanding researchers of electrochemical phase formation processes, whose groundbreaking and rigorous studies were a continuous source of inspiration for our work in this field throughout several decades.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vidal-García, G., Aldana-González, J., Romero-Romo, M. et al. On the transition from 2 to 3D nucleation during the potentiostatic Zn electrodeposition from reline deep eutectic solvent. J Solid State Electrochem 28, 1631–1639 (2024). https://doi.org/10.1007/s10008-023-05623-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05623-6