Abstract
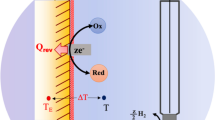
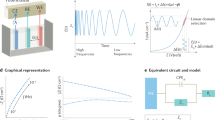
Electron transfer reactions are usually accompanied by heat generated as a byproduct. This heat is the sum of reversible effects as the molar electrochemical Peltier heat and irreversible effects like overpotential and the Joule effect. These effects have been calculated by measuring temperature changes in the working electrode, using calorimetric and electrochemical techniques involving direct current. This work presents a theoretical-experimental strategy to calculate for the first time two new thermometric transfer functions: Gibbs free energy, \(\Delta G(\omega )\), and enthalpy changes, \(\Delta H(\omega )\). Electrochemical impedance spectroscopy, EIS, and other transfer functions, including variation of interfacial temperature VIT, molar electrochemical Peltier heat \(\Pi (\omega )\), and entropy change \(\Delta S(\omega )\) are used to develop the theoretical experimental strategy and calculated the two new thermometric transfer functions. The theoretical models for computing \(\Delta G(\omega )\) and \(\Delta H(\omega )\) were validated with previously experimental reported data of VIT, \(\Pi (\omega )\) and \(\Delta S(\omega )\) for the ferrocyanide/ferricyanide electrochemical system. Nyquist and Bode diagrams for \(\Delta G(\omega )\) and \(\Delta H(\omega )\) are shown, and a brief discussion concerning their behavior is presented.






Similar content being viewed by others
References
Inzelt G (2015) Crossing the bridge between thermodynamics and electrochemistry. From the potential of the cell reaction to the electrode potential. ChemTexts. https://doi.org/10.1007/s40828-014-0002-9
Bard AJ, Inzelt G, Scholz F (2008) Electrochemical dictionary. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-74598-3
Breitkopf C, Swider-Lyons K (2017) Springer handbook of electrochemical energy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46657-5
Bárcena-Soto M, Scholz F (2002) The thermodynamics of the insertion electrochemistry of solid metal hexacyanometallates. J Electroanal Chem. https://doi.org/10.1016/S0022-0728(02)00710-6
Bárcena-Soto M, Kubsch G, Scholz F (2002) Cyclic voltammetry of immobilized microparticles with in situ calorimetry: part I: the thermistor electrode. J Electroanal Chem. https://doi.org/10.1016/S0022-0728(02)00892-6
Vetter KJ (1967) Electrochemical thermodynamics. In: Electrochemical kinetic. Elsevier. https://doi.org/10.1016/B978-1-4832-2936-2.50005-6
Scholz F (2010) Thermodynamics of electrochemical reactions, electroanalytical methods, Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-02915-8_2
González-Meza OA, Larios-Durán ER, Gutiérrez-Becerra A, Casillas N, Escalante JI, Bárcena-Soto M (2019) Development of a Randles-Ševčík-like equation to predict the peak current of cyclic voltammetry for solid metal hexacyanoferrates. J Solid State Electrochem. https://doi.org/10.1007/s10008-019-04410-6
Holmes HF, Joncich MJ (1959) Thermal electroanalysis. Anal Chem. https://doi.org/10.1021/ac60145a006
Graves BB (1972) Differential voltammetric scanning thermometry of tenth formal formaldehyde solution in formal perchloric acid. Anal Chem. https://doi.org/10.1021/ac60314a003
Franklin TC, McCrea R (1974) Heat effects, another method of studying electrodeposition processes. J Electroanal Chem Interfacial Electrochem. https://doi.org/10.1016/0300-9416(74)90034-0
Tamamushi R (1975) The electrochemical Peltier effect observed with electrode reactions of Fe(II)/Fe(III) redox couples at a gold electrode. J Electroanal Chem. https://doi.org/10.1016/0368-1874(75)85122-7
Ozeki T, Watanabe I, Ikeda S (1979) The application of the thermistor-electrode to Peltier heat measurement. J Electroanal Chem Interfacial Electrochem. https://doi.org/10.1016/S0022-0728(79)80308-3
Donepudi VS, Conway BE (1984) Electrochemical calorimetry of the zinc and bromine electrodes in zinc-bromine and zinc-air batteries. J Electrochem Soc. https://doi.org/10.1149/1.2115877
Shibata S, Sumino MP, Yamada A (1985) An improved heat-responsive electrode for the measurement of electrochemical Peltier heat. The Peltier heat for electrosorption and electrodesorption of oxygen on a platinized platinum electrode in sulfuric acid solution. J Electroanal Chem. https://doi.org/10.1016/0022-0728(85)85057-9
Boudeville P (1994) Thermometric determination of electrochemical Peltier heat (thermal effect associated with electron transfer) of some redox couples. Inorganica Chim Acta. https://doi.org/10.1016/0020-1693(94)04072-9
Boudeville P, Tallec A (1988) Electrochemistry and calorimetry coupling: IV. Determination of electrochemical peltier heat. Thermochim Acta. https://doi.org/10.1016/0040-6031(88)87268-X
Fang Z (2011) Some basic matters on the heat effects at electrode-electrolyte interfaces. Thermochim Acta. https://doi.org/10.1016/j.tca.2011.01.017
Sanchez-Amaya M, Bárcena-Soto M, Rodríguez-López A, Antaño-López R, Larios-Durán ER (2020) Sinusoidal temperature variation response associated with electrochemical Peltier heat as a transfer function approach. Electrochem Commun. https://doi.org/10.1016/J.ELECOM.2020.106769
Sánchez-Amaya M, Bárcena-Soto M, Antaño-López R, Rodríguez-López A, Gutiérrez-Becerra A, Larios-Durán ER (2021) Frequency responses of molar electrochemical Peltier heat and entropy changes analyzed as thermometric transfer functions. J Electrochem. https://doi.org/10.1149/1945-7111/AC38F3
Sanchez-Amaya M, Bárcena-Soto M, Antaño-López R, Rodríguez-López A, Barragan, J A, Gutiérrez-Becerra A, Larios-Durán ER (2022) Effect of wide ranges of polarization and concentration on the behavior of ferricyanide/ferrocyanide systems studied through electrochemical measurements. Int J Electrochem Sci. https://doi.org/10.20964/2022.01.11
Niwa K, Doblhofer K (1986) IR spectroscopic study of adsorbed species formed on electrodes during the Fe(CN)3–4−6 charge transfer reaction. Electrochim Acta. https://doi.org/10.1016/0013-4686(86)80106-2
Zhang D, Wang K, Sun D, Xia X, Chen H (2003) Potentiodynamic deposition of Prussian blue from a solution containing single component of ferricyanide and its mechanism investigation. J Solid State Electrochem. https://doi.org/10.1007/s10008-003-0420-x
Karyakin AA (2001) Prussian blue and its analogues: electrochemistry and analytical applications. Electroanalysis. https://doi.org/10.1002/1521-4109(200106)13:10%3c813::AID-ELAN813%3e3.0.CO;2-Z
Larios-Durán ER, Antaño-López R, Keddam M, Meas Y, Takenouti H, Vivier V (2010) Dynamics of double-layer by AC modulation of the interfacial capacitance and associated transfer functions. Electrochim Acta. https://doi.org/10.1016/j.electacta.2009.10.036
Antaño-Lopez R, Keddam M, Takenouti H (2001) A new experimental approach to the time-constants of electrochemical impedance: frequency response of the double layer capacitance. Electrochim Acta. https://doi.org/10.1016/S0013-4686(01)00640-5
Levine IN (2014) Physical chemistry. McGraw-Hill, New York
Bouty M (1879) Sur un phénomène analogue au phénomène de Peltier. J Phys Theor Appl. https://doi.org/10.1051/jphystap:018790080034101
Gottfried JM, Schuster R (2016) Surface microcalorimetry. Surf Interfaces. https://doi.org/10.1002/9783527680573.CH32
Orazem ME, Tribollet B (2017) Electrochemical impedance spectroscopy. JWS. https://doi.org/10.1002/9781119363682
Angell DH, Dickinson T (1972) The kinetics of the ferrous/ferric and ferro/ferricyanide reactions at platinum and gold electrodes. J Electroanal Chem Interfacial Electrochem. https://doi.org/10.1016/S0022-0728(72)80294-8
Acknowledgements
The authors thank CONACyT for the financial support to the project CF-2096004. Hernández-Rizo acknowledges the master’s grant given by CONACyT.
Funding
This work was supported by CONACyT through project CF-2096004 and Hernández-Rizo master’s grant.
Author information
Authors and Affiliations
Contributions
S. G. Hernández-Rizo: investigation, methodology, validation, writing—original draft; M. Bárcena-Soto: conceptualization, methodology, writing—review and editing, visualization, supervision, project administration; E.R. Larios-Durán: conceptualization, resources, writing—original draft, review and editing, visualization, supervision, project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hernández-Rizo, S.G., Larios-Durán, E.R. & Bárcena-Soto, M. Frequency response of Gibbs free energy and enthalpy changes of electrochemical systems analyzed as thermometric transfer functions. J Solid State Electrochem 27, 3177–3188 (2023). https://doi.org/10.1007/s10008-023-05553-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05553-3