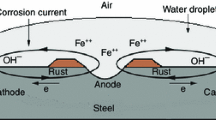
Abstract
The copolymer poly (aniline-co-orthotoluidine) noted poly (ANI-co-OT) was chemically synthesized and characterized by FT-IR, UV–Vis, and XRD techniques. XRD results confirm the amorphous nature of the copolymer. FT-IR and UV–Vis results indicate that the spectrum of the copolymer includes all the bands relating to the functional groups of the homopolymers polyaniline (PANI) and poly-orthotoluidine (POT). It was revealed that, contrastingly to the copolymer’s solubility in dimethylformamide (DMF), the homopolymers have a low solubility. The potentiodynamic polarization technique has been employed in order to study this copolymer’s inhibition effects on the corrosion of carbon steel X52 in a 3.5% NaCl solution. The aforementioned study illuminated the following. The copolymer exhibits high inhibition activity towards the corrosive action of NaCl and its adsorption obeys the Langmuir adsorption isotherm model. The calculated Gibbs free energy (∆G0ads) revealed the chemisorption of this copolymer on the surface of the carbon steel. In addition, a synergistic effect was observed when the copolymer poly (ANI-co-OT) was mixed with the amphoteric surfactant cocamidopropyl betaine (CAPB) where the inhibition efficiency increased from 68 to 92%. Also, it was perceived that the adsorption of the copolymer/surfactant mixture adhered to the Langmuir adsorption isotherm. The calculated Gibbs free energy (∆G0ads) revealed both chemisorption and physisorption of the mixed copolymer and surfactant on the carbon steel surface. Increasing temperature slightly decreases the inhibition efficiency, indicating that the mixed copolymer and surfactant adsorb on the carbon steel surface via simultaneous chemisorption and physisorption. The good inhibition efficiency was related to the formation of inhibitor–adsorption film on the surface of the carbon steel, which is confirmed by the surface analysis. Quantum chemical results using the density functional theory (DFT) corroborated the experimental results.


















Similar content being viewed by others
References
Moulai F, Hadjersi T, Achour A (2021) The effect of zinc shape on its corrosion mitigation as an anode in aqueous Zn/MnO2 battery. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2021.115140
Benchikh A, Aitout R, Makhloufi L, Benhaddad L, Saidani B (2009) Soluble conducting poly(aniline-co-orthotoluidine) copolymer as corrosion inhibitor for carbon steel in 3% NaCl solution. Desalination 249:466–474
Dadgarinezhad A, Baghaei F (2009) The inhibition mild steel corrosion in phosphoric acid solutions by 2-phenyl-1-hydrazine carboxamide. J Chil Chem Soc 54:208–211
Keny SJ, Kumbhar AG, Thinaharan C, Venkateswaran G (2008) Gallic acid as a corrosion inhibitor of carbon steel in chemical decontamination formulation. Corros Sci 50:411–419
Müller B, Fischer S (2006) Epoxy ester resins as corrosion inhibitors for aluminium and zinc pigments. Corros Sci 48:2406–2416
Singh Bisht BM, Bhandari H, Gairola SP, Dhawan SK (2016) Development of conducting copolymer based on poly (o-toluidine-co-2-amino 5 napthol 7 sulphonic acid): an efficient material for protection of iron in highly corrosive environment. Int J Res Eng Technol 5:44–51
Mohd R, Suhail S, Afidah AR, Umesh W (2014) Polyaniline/palm oil blend for anticorrosion of mild steel in saline environment. J Appl Chem. https://doi.org/10.1155/2014/973653
Bhandari H, Choudhary V, Dhawan SK (2008) Synergistic effect of copolymers composition on the electrochemical, thermal, and electrical behavior of 5-lithiosulphoisophthalic acid doped poly(aniline-co-2-isopropylaniline): synthesis, characterization, and applications. Polym Adv Technol 20:1024–1034
Yagan A, Pekmez NO, Yildiz A (2006) Corrosion inhibition by poly(N-ethylaniline) coatings for mild steel in aqueous acidic solutions. Prog Org Coat 57:314–318
Hung HM, Linh DK, Chinh NT, Duc LM, Trung VQ (2019) Improvement of the corrosion protection of polypyrrole coating for CT3 mild steel with 10-camphorsulfonic acid and molybdate as inhibitor dopants. Prog Org Coat 131:407–416
Gvozdenović M, Džunuzović E, Jugović B, Grgur B (2018) Polyaniline based corrosion inhibitors for conventional organic coatings. Zastita Materijala 59:282–292
Nguyen MT, Diaz AF (1995) Water-soluble poly(aniline-co-o-anthranilic acid) copolymers. Macromolecules 28:3411–3415
Yong L, Huixia F, Hou X, Wu HX (2022) Carboxymethyl cellulose polyaniline composites as efficient corrosion inhibitor for Q235 steel in 1M HCl solution. Int J Electrochem. https://doi.org/10.20964/2022.11.69
Kong P, Feng H, Chen N, Lu Y, Li S, Wang P (2019) Polyaniline/chitosan as a corrosion inhibitor for mild steel in acidic medium. RSD Adv 9:9211–9217
Lei Y, Qiu Z, Tan N, Du H, Li D, Liu J, Liu T, Zhang W, Chang X (2020) Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on caron steel in 3.5% NaCl solution. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2019.105430
Ilassi K, Bahgat A, Sadasivuni K K, Mrlik M (2018) Anti-corrosive and oil sensitive coatings based on epoxy/polyaniline/magnetite-clay composites through diazonium interfacial chemistry. Sci Rep. https://doi.org/10.1038/s41598-018-31508-0
Dhawan SK, Trivedi DC (1993) Influence of polymerization conditions on the properties of poly(2-methylaniline) and its copolymer with aniline. Synth Met 60:63–66
Zhang F, Li X, Deng S, Tang M, Du G (2021) Amphoteric surfactant of octadecyl dimethyl betaine as an efficient corrosion inhibitor for cold rolled steel in phosphoric acid solution. J Mater Res Technol 15:7050–7069
Zhuang W, Wang X, Zhu W, Zhang Y, Sun D, Zhang R, Wu C (2021) Imidazoline gemini surfactants as corrosion inhibitors for carbon steel X70 in NaCl solution. ACS Omega 6:5653–5660
El Ibrahimi B, Jmiai A, Bazzi L, El Issami S (2020) Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab J Chem 13:740–771
Jacob SE, Amini S (2008) Cocamidopropyl betaine. Dermatitis 19:157–160
Basheva ES, Ganchev DN, Denkov D, Kasuga K, Satoh N, Tsujii K (2000) Role of betaine as foam booster in the presence of silicone oil drops. Langmuir 16:1000–1013
Fowler JF, Zug KM, Tylor JS, Storrs FJ, Sherertz EA, Sasseville DA, Rietschel RL, Pratt MD, Mathia CGT, Marks JG, Maibach HI, Fransway AF, Deleo VA, Belsito DV (2004) Allergy to cocamidopropyl betaine and amidoamine in North America. Dermatitis 15:5–6
Foti C, Bonamonte D, Mascolo G, Corcelli A, Lobasso S, Rigano L, Angelini G (2003) The role of 3-dimethylaminopropylamine and amidoamine in contact allergy to cocoamidopropylbetaine. Contact Dermat 48:194–198
Mobin M, Khan MA (2011) Adsorption and corrosion inhibition behavior of polyethylene glycol and surfactants additives on mild steel in H2SO4. J Mater Eng Perform 23:222–229
Mobin M, Khan MA (2013) Synergistic influence of polyvinyl alcohol and surfactants on the corrosion inhibition of mild steel in 0.1 M H2SO4. Chemical Engineering and Communication 200:1149–1169
Yousefi SR, Alshamsi HA, Amiri O, Salavati-Niasari M (2021) Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J Mol Liq 337:116405
Mehdizadeh P, Jamdar M, Mahdi MA, Abdulsahib WK, Jasim LS, Yousefi SR, Salavati-Niasari M (2023) Rapid microwave fabrication of new nanocomposites based on Tb-Co-O nanostructures and their application as photocatalysts under UV/visible light for removal of organic pollutants in water. Arab J Chem 16:104579
Yousefi SR, Sobhani A, Salavati-Niasari M (2017) A new nanocomposite superionic system (CdHgI4/HgI2): synthesis, characterization and experimental investigation. Adv Powder Technol 28:1258–1262
Yousefi SR, Masjedi-Arani M, Morassaei MS, Salavati-Niasari M, Moayedi H (2019) Hydrothermal synthesis of DyMn2O5/Ba3Mn2O8 nanocomposite as a potential hydrogen storage material. Int J Hydrogen Energy 44:24005–24016
Mahdi MA, Yousefi SR, Jasim LS, Salavati-Niasari M (2022) Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: photocatalytic and antibacterial activities. Int J Hydrogen Energy 47:14319–14330. https://doi.org/10.1016/j.ijhydene.2022.02.175
Yousefi SR, Ghanbari M, Amiri O, Marzhoseyni Z, Mehdizadeh P, Hajizadeh-Oghaz M, Salavati-Niasari M (2021) Dy2BaCuO5/Ba4DyCu3O9.09 S-scheme heterojunction nanocomposite with enhanced photocatalytic and antibacterial activities. J Am Ceram Soc 104:2952–2965
Yousefi SR, Sobhani A, Alshamsi HA, Salavati-Niasari M (2021) Green sonochemical synthesis of BaDy2NiO5/Dy2O3 and BaDy2NiO5/NiO nanocomposites in the presence of core almond as a capping agent and their application as photocatalysts for the removal of organic dyes in water. RSC Adv 11:11500–11512
Yousefi SR, Amiri O, Salavati-Niasari M (2019) Control sonochemical parameter to prepare pure Zn0.35Fe2.65O4 nanostructures and study their photocatalytic activity. Ultrason Sonochem 58:104619
Yousefi SR, Ghanbari D, Salavati-Niasari M, Hassanpour M (2016) Photo-degradation of organic dyes: simple chemical synthesis of Ni(OH)2 nanoparticles, Ni/Ni(OH)2 and Ni/NiO magnetic nanocomposites. J Mater Sci: Mater Electron 27:1244–1253
Zulfareen N, Kannan K, Venugopal T, Gnanavel S (2016) Synthesis, characterization and corrosion inhibition efficiency of N-(4-(morpholinomethyl carbamoyl phenyl) furan-2-carboxamide for brass in HCl medium. Arab J Chem 9:121–135
Parr RG, Szentpaly L, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Kokalj A (2010) Is the analysis of molecular electronic structure of corrosion inhibitors sufficient to predict the trend of their inhibition performance. Electrochim Acta 56:745–755
Bhadra S, Kim NH, Rhee KY, Lee JH (2009) Preparation of nanosize polyaniline by solid-state polymerization and determination of crystal structure. Polym Int 58:1173–1180
Oh M, Park SJ, Jung Y, Kim S (2012) Electrochemical properties of polyaniline composite electrodes prepared by in-situ polymerization in titanium dioxide dispersed aqueous solution. Synth Met 162:695–701
Pant HC, Patra MK, Negi SC, Bhatia A, Vadera SR, Kumar N (2006) Studies on conductivity and dielectric properties of polyaniline–zinc sulphide composites. Bull Mater Sci 29:379–384
Reddy KR, Sin BC, Ryu KS, Noh J, Lee Y (2009) In situ self-organization of carbon black-polyaniline composites from nanospheres to nanorods: synthesis, morphology, structure and electrical conductivity. Synth Met 159:1934–1939
Pouget JP, Hsu CH, MacDiarmid AG, Epstein AJ (1995) Structural investigation of metallic PAN-CSA and some of its derivatives. Synth Met 69:119–120
Chaudhari HK, Kelkar DS (1997) Investigation of structure and electrical conductivity in doped polyaniline. Polym Int 42:380–384
Wang Q, Li JL, Gao F, Li WS, Wu KZ, Wang XD (2008) Activated carbon coated with polyaniline as an electrode material in supercapacitors. New Carbon Mater 23:275–280
Padmapriya S, Seshadri H, Jaidev KJ, Venkatachalam S, Kumar D, Pal S (2017) Storage and evolution of hydrogen in acidic medium by polyaniline. Int J Energy Res 42:1196–1209
Vadiraj KT, Belagali SL (2015) Characterization of polyaniline for optical and electrical properties. J Appl Chem 8:53–56
Fuseini M, El-Shazly AH, Elkady MF (2020) Effects of doping on zeta potential and pH of polyaniline colloidal suspension. Mater Sci Forum 1008:114–120
Mostafaei A, Zolriasatein A (2012) Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog Nat Sci 22:273–280
Luzny W, Banka E (2000) Relations between the structure and electric conductivity of polyaniline protonated with camphorsulfonic acid. Macromolecules 33:425–429
Pouget JP, Jozefowicz ME, Epstein AJ, Tang X, MacDiarmid AG (1991) X-ray structure of polyaniline 24(3):779–789. https://doi.org/10.1021/ma00003a022
Zhang J, LI Y, Zhang S, (2018) Preparation, characterization and corrosion evaluation of poly(o-toluidine), poly(m-toluidine), and poly(p-Toluidine) blended with waterborne polyurethane. JOM 70:2660–2666
Reddy KR, Lee KP, Gopalan AI (2007) Self-assembly directed synthesis of poly(ortho-toluidine)-metal (gold and palladium) composite nanospheres. J Nanosci Nanotechnol 7:3117–3125
Khan A, Khan I, Asiri AM (2021) Preparation and characterization of new and novel poly-o-toluidine Sn (II) silicotungstate ternary nanocomposite and its environment application as indicator electrode. J Saudi Chem Soc 25:101385–101392
Ping Z (1996) In situ FTIR-attenuated total reflection spectroscopic investigations on the base–acid transitions of polyaniline. Base-acid transition in the emeraldine form of polyaniline. J Chem Soc Faraday Trans 92:3063–3067
Sedenkova I, Trchova M, Stejskal J (2008) Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water-FTIR and Raman spectroscopic studies. Polym Degrad Stab 93:2147–2157
Song H, Zhang J, Song P, Xiong Y (2019) Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor. e-Polymers 19:313–322
Liu H, Hu XB, Wang JY, Boughton RI (2002) Structure, conductivity, and thermopower of crystalline polyaniline synthesized by the ultrasonic irradiation polymerization method. Macromolecules 35:9414–9419
Gruger A, Novak A, Regis A, Colomban Ph (1994) Infrared and Raman study of polyaniline part ii: influence of ortho substituents on hydrogen bonding and UV/Vis – near – IR electron charge transfer. J Mol Struct 328:153–167
Fujita I, Ishiguchi M, Shiota H, Dani T, Kosai KJ (1992) Spectroscopic study of polytoluidines in the UV-visible and IR regions. J Appl Polym Sci 44:987–992
Savitha P, Sathyanarayama DN (2004) Synthesis and characterization of soluble conducting poly (o-/m-toluidine-co-o-nitroaniline). Synth Metals 145:113–118
Li XG, Wang LX, Jin Y, Zhu ZL, Yang YL (2001) Preparation and identification of a soluble copolymer from pyrrole and o-toluidine. J Appl Polym Sci 82:510–518
Andriianova AN, Biglova YN, Mustafin AG (2020) Effect of structural factors on the physicochemical properties of functionalized polyanilines. RSC Adv 10:7468–7491
Ghobashy MM, Alkhursani SA, Madani M (2018) Radiation-induced nucleation and pH-controlled nanostructure shape of polyaniline dispersed in DMF. Polym Bull 75:5477–5492
Xin LY, Zhang XG, Zhang GQ, Shen CM (2005) Synthesis and characterization of aniline and o-toluidine conducting copolymer microtubes with the template-synthesis method. J Appl Polym Sci 96:1539–1543
Aslam J, Lone IH, Radwan N, Mobin M, Zehra S, Aslam R (2018) Development of poly(aniline-co-o-toluidine)/TiO2 nanocomposite coatings for low carbon steel corrosion in 3.5% NaCl solution. J Adhes Sci Technol 32:2552–2568
Wu HI, Huang JB, Luo XQ (2014) Synthesis and characterization of cocamidopropyl betaine. China Surfactant Detergent & Cosmetics 44:23–25
Carolei L, Gutz IGR (2005) Simultaneous determination of three surfactants and water in shampoo and liquid soap by ATR-FTIR. Talanta 66:118–124
May M (2016) Corrosion behavior of mild steel immersed in different concentrations of NaCl solutions. J Sebha University 15:1–12
Jovancicevic V, Bockris JO’M, (1986) The mechanism of oxygen reduction on iron in neutral solution. J Electrochem Soc 133:1797–1807
Ituen E, Mkpenie V, Ekemini E (2019) Adsorptive Fe-nanoparticles mediated by Musa sapientum peels extract as anticorrosion additive for aqueous oilfield descaling solution. Sci Afr. https://doi.org/10.1016/j.sciaf.2019.e00075
Perdew JP, Wang Y (1992) Accurate and simple representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249
Gopiraman M, Selvakumaran N, Kesavan D, Kim IS, Karvembu R (2012) Chemical and physical interactions of 1-benzoyl-3,3- disubstituted thiourea derivatives on mild steel surface: corrosion inhibition in acidic media. Ind Eng Chem Res 51:7910–7922
Ituen E, Akaranta O, James A (2017) Evaluation of performance of corrosion inhibitors using adsorption isotherm models: an overview. Chem Sci Int J. https://doi.org/10.9734/CSIJ/2017/28976
Badiea AM, Mohana KN (2009) Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2-hydrazino-4,7-dimethylbenzothiazole in industrial water medium. Corros Sci 51:2231–2241
Umoren SA, Banera MJ, Alonso-Garcia T, Gervasi CA, Mirifico MV (2013) Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 20:2529–2545
Tao Z, He W, Wang S, Zhang S, Zhou GA (2012) Study of differential polarization curves and thermodynamic properties for mild steel in acidic solution with nitropenyltriazole derivative. Corros Sci 60:205–213
Arab ST (2008) Inhibition action of thiosemicabazone and some of it is ρ-substituted compounds on the corrosion of iron-base metallic glass alloy in 0.5 M H2SO4 at 30°C. Mater Res Bull 43:510–521
Wang X, Yang H, Wang F (2010) A cationic gemini-surfactant as effective inhibitor for mild steel in HCl solutions. Corros Sci 52:1268–1276
Li SL, Wang YG, Chen SH, Yu R, Lei SB, Ma HY, Liu DX (1999) Some aspects of quantum chemical calculations for the study of Schiff base corrosion inhibitors on copper in NaCl solutions. Corros Sci 41:1769–1782
Usman J, Umoren SA, Gasem ZM (2017) Inhibition of API 5L X60 steel corrosion in CO2-saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J Mol Liq 237:146–156
Oguzie EE, Li Y, Wang SG, Wang F (2011) Understanding corrosion inhibition mechanisms—experimental and theoretical approach. RSC Adv 1:866–873
Okafor PC, Zheng Y (2009) Synergistic inhibition behaviour of methylbenzene quaternary imidazoline derivative and iodide ions on mild steel in H2SO4 solutions. Corros Sci 51:850–859
Yan Q, Yin Q, Cui J, Wang X, Qiao Y, Zhou H (2021) Effect of temperature on corrosion behaviour of E690 steel in 3.5 wt. % NaCl solution. Mater Res Express. https://doi.org/10.1088/2053-1591/abda69
Yuan W, Huang F, Liu J, Hu Q, Franck Cheng Y (2018) Effects of temperature and applied strain on corrosion of X80 pipeline steel in chloride solutions. Corros Eng Sci Technol 53:393–402
Khadom AA (2014) Effect of temperature on corrosion inhibition of copper-nickel alloy by tetraethylenepentamine under flow conditions. J Chil Chem Soc. https://doi.org/10.4067/S0717-97072014000300004
Szauer T, Brand A (1981) Adsorption of oleates of various amines on iron in acidic solution. Electrochim Acta 26:1253–1256
Hamani H, Douadi T, Daoud D, Al-Noaimi M, Rikkouh RA, Chafaa S (2017) 1-(4-Nitrophenylo-imino)-1-(phenylhydrazono)-propan-2-one as corrosion inhibitor for mild steel in 1 M HCl solution: weight loss, electrochemical, thermodynamic and quantum chemical studies. J Electroanal Chem 801:425–438
Olen L, Riggs JR, Hurd RM (1967) Temperature coefficient of corrosion inhibition. Corrosion 23:252–260
Liu J, Zheng T, Wang J, Jia G (2022) The inhibition performance of novel amino acid-based amphiprotic surfactants on aluminum alloys in sodium chloride solutions: experimental and theoretical studies. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2021.125622
Olasunkanmi LO, Moloto BP, Obot IB, Ebenso EE (2018) Anticorrosion studies of some hydantoin derivatives for mild steel in 0.5 M HCl solution: experimental, quantum chemical, Monte Carlo simulations and QSAR studies. J Mol Liq 252:62–74
Kouache A, Khelifa A, Boutoumi H, Moulay S, Feghoul A, Idir B, Aoudj S (2021) Experimental and theoretical studies of Inula viscosa extract as a novel eco-friendly corrosion inhibitor for carbon steel in 1 M HCl. J Adhes Sci Technol. https://doi.org/10.1080/01694243.2021.195621
Lukovits I, Kalman E, Zucchi F (2001) Corrosion inhibitors -correlation between electronic structure and efficiency. Corrosion 57:3–8
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Copolymer and amphoteric surfactant show high corrosion inhibition performance of carbon steel in 3.5%NaCl solution.
• Both inhibitors are mixed-type ones, obeying the Langmuir adsorption isotherm.
• The synergistic effect is more pronounced by increasing the amount of surfactant.
• Theoretical studies are in good agreement with experimental results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Djama, M., Benhaddad, L., Idir, B. et al. Synergistic corrosion inhibition effect of copolymer and an amphoteric surfactant on carbon steel in 3.5 NaCl solution: experimental and theoretical research. J Solid State Electrochem 27, 2139–2162 (2023). https://doi.org/10.1007/s10008-023-05456-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05456-3