Abstract
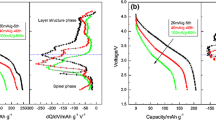
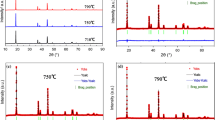
Lithium-rich oxides (Li1.2Ni0.2Mn0.6O2) were obtained by two synthesis routes: co-precipitation method and solid-state reaction. Both materials showed a high degree of crystallinity, and XRD analysis revealed intense and well-defined signals corresponding to the R3m and C2/m space groups of these types of compounds, with a difference in the cationic order in the hexagonal structure layers. The cycling performances showed an initial discharge capacity of 200 mAh g−1 from the co-precipitated material, against the 150 mAh g−1 obtained from the solid-state reaction route but, unlike the large drop in the discharge capacity of the co-precipitated material after 160 cycles, the material obtained by solid-state reaction provided a slightly constant discharge capacity of ⁓120 mAh g−1 throughout cycling. The high initial discharge capacity of the co-precipitated material may be associated with the activation of the Li2MnO3 phase cycled at 0.2 C between 2.0–4.8 V and 2.0–5.2 V, the better cationic order and wider space between the layers of the LiMO2 phase. Therefore, the electrochemical performance could be directly related to those structural characteristics obtained thorough the selected synthetic procedures.












Similar content being viewed by others
References
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195:2419–2430. https://doi.org/10.1016/j.jpowsour.2009.11.048
Xiang X, Li W (2015) Facile synthesis of lithium-rich layered oxide Li[Li0.2Ni0.2Mn0.6]O2 as cathode of lithium-ion batteries with improved cyclic performance. J Solid State Electrochem 19:221–227. https://doi.org/10.1007/s10008-014-2590-0
Dou S (2013) Review and prospect of layered lithium nickel manganese oxide as cathode materials for Li-ion batteries. J Solid State Electrochem 17:911–926. https://doi.org/10.1007/s10008-012-1977-z
Placke T, Kloepsch R, Dühnen S, Winter M (2017) Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J Solid State Electrochem 21:1939–1964. https://doi.org/10.1007/s10008-017-3610-7
Diouf B, Pode R (2015) Potential of lithium-ion batteries in renewable energy. Renew Energy 76:375–380. https://doi.org/10.1016/j.renene.2014.11.058
Liu Q, Su X, Lei D et al (2018) Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat Energy 3:936–943. https://doi.org/10.1038/s41560-018-0180-6
Li M, Wang H, Zhao L et al (2019) Improving the electrochemical performance of lithium-rich oxide layer material with Mg and La co-doping. J Alloys Compd 782:451–460. https://doi.org/10.1016/j.jallcom.2018.12.072
Pan H, Zhang S, Chen J et al (2018) Li- and Mn-rich layered oxide cathode materials for lithium-ion batteries: a review from fundamentals to research progress and applications. Mol Syst Des Eng 3:748–803. https://doi.org/10.1039/C8ME00025E
Gu R-M, Yan S-Y, Sun S et al (2015) Electrochemical behavior of lithium-rich layered oxide Li[Li0.23Ni0.15Mn0.62]O2 cathode material for lithium-ion battery. J Solid State Electrochem 19:1659–1669. https://doi.org/10.1007/s10008-015-2796-9
Qing R-P, Shi J-L, Xiao D-D et al (2016) Cathode materials: enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping (Adv. Energy Mater. 6/2016). Adv Energy Mater 6. https://doi.org/10.1002/aenm.201670035
Lu C, Wu H, Zhang Y et al (2014) Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J Power Sources 267:682–691. https://doi.org/10.1016/j.jpowsour.2014.05.122
Watanabe A, Yamamoto K, Uchiyama T et al (2020) Capacity improvement by nitrogen doping to lithium-rich cathode materials with stabilization effect of oxide ions redox. ACS Appl Energy Mater 3:4162–4167. https://doi.org/10.1021/acsaem.0c00564
Zhang K, Sheng H, Wu X et al (2020) Improving electrochemical properties by sodium doping for lithium-rich layered oxides. ACS Appl Energy Mater 3:8953–8959. https://doi.org/10.1021/acsaem.0c01402
Zhang J, Lu Q, Fang J et al (2014) Polyimide encapsulated lithium-rich cathode material for high voltage lithium-ion battery. ACS Appl Mater Interfaces 6:17965–17973. https://doi.org/10.1021/am504796n
He W, Qian J, Cao Y et al (2012) Improved electrochemical performances of nanocrystalline Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. RSC Adv 2:3423–3429. https://doi.org/10.1039/C2RA20122D
Koga H, Croguennec L, Ménétrier M et al (2013) Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J Electrochem Soc 160:A786. https://doi.org/10.1149/2.038306jes
Liu Y, Wang Q, Zhang Z et al (2016) Investigation the electrochemical performance of layered cathode material Li 1.2 Ni 0.2 Mn 0.6 O 2 coated with Li 4 Ti 5 O 12. Adv Powder Technol 27:1481–1487. https://doi.org/10.1016/j.apt.2016.05.008
Xu J, Sun M, Qiao R et al (2018) Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat Commun 9:947. https://doi.org/10.1038/s41467-018-03403-9
Hamad KI, Xing Y (2019) Stabilizing Li-rich NMC materials by using precursor salts with acetate and nitrate anions for Li-ion batteries. Batteries 5:69. https://doi.org/10.3390/batteries5040069
Hu S, AnoopS P, Liang G et al (2019) Li-rich layered oxides and their practical challenges: recent progress and perspectives. Electrochem Energy Rev 2:277–311. https://doi.org/10.1007/s41918-019-00032-8
Song X, Huang H, Zhong W (2019) Sucrose-assisted synthesis of layered lithium-rich oxide Li[Li0.2Mn0.56Ni0.16Co0.08]O2 as a cathode of lithium-ion battery. Crystals 9:436. https://doi.org/10.3390/cryst9090436
Ohzuku T, Ueda A, Nagayama M (1993) Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J Electrochem Soc 140:1862–1870. https://doi.org/10.1149/1.2220730
An J, Shi L, Chen G et al (2017) Insights into the stable layered structure of a Li-rich cathode material for lithium-ion batteries. J Mater Chem A 5:19738–19744. https://doi.org/10.1039/C7TA05971J
Lu Z, Beaulieu LY, Donaberger RA et al (2002) Synthesis, structure, and electrochemical behavior of Li [Ni x Li1 / 3–2x / 3Mn2 / 3–x / 3 ] O 2. J Electrochem Soc 149:A778. https://doi.org/10.1149/1.1471541
Ates MN, Mukerjee S, Abraham KM (2015) A high rate Li-rich layered MNC cathode material for lithium-ion batteries. RSC Adv 5:27375–27386. https://doi.org/10.1039/C4RA17235C
He X, Wang J, Wang L, Li J (2016) Nano-crystalline Li1.2Mn0.6Ni0.2O2 prepared via amorphous complex precursor and its electrochemical performances as cathode material for lithium-ion batteries. Materials 9:661. https://doi.org/10.3390/ma9080661
Abdel-Ghany A, Hashem AM, Mauger A, Julien CM (2020) Lithium-rich cobalt-free manganese-based layered cathode materials for Li-ion batteries: suppressing the voltage fading. Energies 13:3487. https://doi.org/10.3390/en13133487
Wang C-C, Lin Y-C, Chou P-H (2015) Mitigation of layer to spinel conversion of a lithium-rich layered oxide cathode by substitution of Al in a lithium ion battery. RSC Adv 5:68919–68928. https://doi.org/10.1039/C5RA11665A
Li Y, Li Z, Chen C et al (2021) Recent progress in Li and Mn rich layered oxide cathodes for Li-ion batteries. J Energy Chem 61:368–385. https://doi.org/10.1016/j.jechem.2021.01.034
Yu F-D, Que L-F, Wang Z-B et al (2017) Controllable synthesis of hierarchical ball-in-ball hollow microspheres for a high performance layered Li-rich oxide cathode material. J Mater Chem A 5:9365–9376. https://doi.org/10.1039/C7TA02553J
A.Said AE-A, El-Wahab MMA, Soliman SA, Goda MN (2014) Synthesis and characterization of nano CuO-NiO mixed oxides. Nanosci Nanoeng 2:17–28. https://doi.org/10.13189/nn.2014.020103
Stella C, Soundararajan N, Ramachandran K (2014) Structural, optical, dielectric and magnetic properties of Mn1−xCoxO2 nanowires. Superlattices Microstruct 71:203–210. https://doi.org/10.1016/j.spmi.2014.03.044
Bizeau J, Tapeinos C, Marella C et al (2017) Synthesis and characterization of hyaluronic acid coated manganese dioxide microparticles that act as ROS scavengers. Colloids Surf B Biointerfaces 159:30–38. https://doi.org/10.1016/j.colsurfb.2017.07.081
Pasierb P, Komornicki S, Rokita M, Rȩkas M (2001) Structural properties of Li2CO3–BaCO3 system derived from IR and Raman spectroscopy. J Mol Struct 596:151–156. https://doi.org/10.1016/S0022-2860(01)00703-7
Bunaciu AA, Udriştioiu EG, Aboul-Enein HY (2015) X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem 45:289–299. https://doi.org/10.1080/10408347.2014.949616
Ito K, Bernstein HJ (1956) The vibrational spectra of the formate, acetate, and oxalate ions. Can J Chem 34:170–178. https://doi.org/10.1139/v56-021
Li J, Zhan C, Lu J et al (2015) Improve first-cycle efficiency and rate performance of layered-layered Li1.2Mn0.6Ni0.2O2 using oxygen stabilizing dopant. ACS Appl Mater Interfaces 7:16040–16045. https://doi.org/10.1021/acsami.5b04343
Uzun D (2015) Boron-doped Li1.2Mn0.6Ni0.2O2 as a cathode active material for lithium ion battery. Solid State Ion 281:73–81. https://doi.org/10.1016/j.ssi.2015.09.008
Yang C-C, Liao P-C, Wu Y-S, Lue SJ (2017) Electrochemical performance of Li-rich oxide composite material coated with Li0.75La0.42TiO3 ionic conductor. Appl Surf Sci 399:670–681. https://doi.org/10.1016/j.apsusc.2016.12.121
Chen H, Islam MS (2016) Lithium extraction mechanism in Li-Rich Li2MnO3 involving oxygen hole formation and dimerization. Chem Mater 28:6656–6663. https://doi.org/10.1021/acs.chemmater.6b02870
Mezaal MA, Qu L, Li G et al (2017) LiMO2@Li2MnO3 positive-electrode material for high energy density lithium ion batteries. J Solid State Electrochem 21:145–152. https://doi.org/10.1007/s10008-016-3345-x
Hu J-P, Sheng H, Deng Q et al (2020) High-rate layered cathode of lithium-ion batteries through regulating three-dimensional agglomerated structure. Energies 13:1602. https://doi.org/10.3390/en13071602
Zhao S, Yan K, Zhang J et al (2021) Reaction mechanisms of layered lithium-rich cathode materials for high-energy lithium-ion batteries. Angew Chem Int Ed 60:2208–2220. https://doi.org/10.1002/anie.202000262
Abdellahi A, Urban A, Dacek S, Ceder G (2016) Understanding the effect of cation disorder on the voltage profile of lithium transition-metal oxides. Chem Mater 28:5373–5383. https://doi.org/10.1021/acs.chemmater.6b01438
Li L, Lee KS, Lu L (2014) Li -rich layer-structured cathode materials for high energy Li -ion batteries. Funct Mater Lett 07:1430002. https://doi.org/10.1142/S1793604714300023
Zhang X, Meng X, Elam JW, Belharouak I (2014) Electrochemical characterization of voltage fade of Li1.2Ni0.2Mn0.6O2 cathode. Solid State Ion 268:231–235. https://doi.org/10.1016/j.ssi.2013.09.052
Kim D, Sandi G, Croy JR et al (2013) Composite ‘Layered-Layered-Spinel’ cathode structures for lithium-ion batteries. J Electrochem Soc 160:A31–A38. https://doi.org/10.1149/2.049301jes
Boulineau A, Simonin L, Colin J-F et al (2013) First evidence of manganese–nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett 13:3857–3863. https://doi.org/10.1021/nl4019275
Xie H, Cui J, Yao Z et al (2022) Revealing the role of spinel phase on Li-rich layered oxides: a review. Chem Eng J 427:131978. https://doi.org/10.1016/j.cej.2021.131978
Li X, Xu Y, Wang C (2009) Suppression of Jahn-Teller distortion of spinel LiMn2O4 cathode. J Alloys Compd 479:310–313. https://doi.org/10.1016/j.jallcom.2008.12.081
Yang Z, Zheng C, Wei Z et al (2022) Multi-dimensional correlation of layered Li-rich Mn-based cathode materials. Energy Mater. https://doi.org/10.20517/energymater.2022.02
Xiong L, Sun M, Xu Y et al (2018) Synthesis of carbon coated Li2MnO3 cathode material with enhanced rate capability for lithium-ion batteries. Solid State Ion 325:170–175. https://doi.org/10.1016/j.ssi.2018.08.008
Zhang Y, Liu Z, Wang Z et al (2019) Electrochemical impedance spectroscopy study of lithium-rich material 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 in the first two charge-discharge cycles. Electrochim Acta 310:136–145. https://doi.org/10.1016/j.electacta.2019.04.112
Liu Y, Lv J, Liu S et al (2013) Improved electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode materials by ball milling and carbon coating. Powder Technol 239:461–466. https://doi.org/10.1016/j.powtec.2013.02.039
He W, Zheng H, Ju X et al (2017) Multistage Li 1.2 Ni 0.2 Mn 0.6 O 2 micro-architecture towards high-performance cathode materials for lithium-ion batteries. Chem Electro Chem 4:3250–3256. https://doi.org/10.1002/celc.201700727
Lu Y, Pang M, Shi S et al (2018) Enhanced electrochemical properties of Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 cathode material for lithium-ion battery at elevated temperature. Sci Rep 8:2981. https://doi.org/10.1038/s41598-018-21345-6
Chen Y, Wang X, Zhang J et al (2019) Al 2 O 3 -coated Li 1.2 Mn 0.54 Ni 0.13 Co 0.13 O 2 nanotubes as cathode materials for high-performance lithium-ion batteries. RSC Adv 9:2172–2179. https://doi.org/10.1039/C8RA09428D
Zhao T, Zhou N, Zhang X et al (2017) Structure evolution from layered to spinel during synthetic control and cycling process of Fe-containing Li-rich cathode materials for lithium-ion batteries. ACS Omega 2:5601–5610. https://doi.org/10.1021/acsomega.7b00689
Fu P, Lu W, Lei W et al (2013) Thermal stability and microstructure characterization of MgAl2O4 nanoparticles synthesized by reverse microemulsion method. Mater Res 16:844–849. https://doi.org/10.1590/S1516-14392013005000062
Hou XL, Huang JT, Hu ZH et al (2018) Molten salt synthesis and formation mechanism of plate-like MgAl2O4 spinel. Solid State Phenom 281:278–284. https://doi.org/10.4028/www.scientific.net/SSP.281.278
Shimoda K, Oishi M, Matsunaga T et al (2017) Direct observation of layered-to-spinel phase transformation in Li 2 MnO 3 and the spinel structure stabilised after the activation process. J Mater Chem A 5:6695–6707. https://doi.org/10.1039/C6TA11151C
Acknowledgements
This work was supported by the CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas) and ANPCyT (Agencia Nacional de Promoción Científica y Tecnológica).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodriguez, A., Sanservino, M.A., Gómez, S. et al. Effect of co-precipitation and solid-state reaction synthesis methods on lithium-rich cathodes Li1.2Ni0.2Mn0.6O2. J Solid State Electrochem 26, 2315–2328 (2022). https://doi.org/10.1007/s10008-022-05258-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05258-z