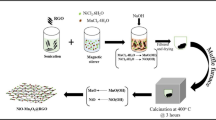
Abstract
Graphene (G) and ternary nanocomposites of Mn3O4, TiO2, and reduced graphene oxide(rGO) electrodes have been prepared for supercapacitor applications. The as-synthesized samples were characterized using several techniques including XRD, SEM, TEM, XPS, and Raman spectroscopy. Electrochemical characterizations were studied via cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS). XRD patterns of TiO2 and Mn3O4 showed the formation of anatase and hausmannite tetragonal nanoparticles, respectively, whereas rGO and G showed an amorphous structure. The TEM analysis showed spherical shaped particles with less than 50 nm sizes for Mn3O4, nanotube for TiO2, fiber structure for rGO, and layered structure for graphene. The Mn3O4/TiO2/rGO ternary nanocomposite electrode presented a much higher specific capacitance than its single individual constituents. The ternary nanocomposite has a specific capacitance of 356 F g−1 in 6 M KOH aqueous electrolyte and respectable cycling performance, with 91% capacitance retained over 3000 cycles referring to its suitability for supercapacitor applications. An asymmetric supercapacitor (ASC) was constructed using a Mn3O4–TiO2–rGO (MTrGO) as a positive electrode and G as a negative electrode. The organized (ASC) works steadily under the potential window of 0–1.8 V and provides a high-energy density of 31.95 Wh kg−1 at a power density of 7188 W kg−1 complemented by satisfactory cycle stability with 87% capacitance retention over 1000 cycles.

Graphical Abstract










Similar content being viewed by others
References
Aray A, Sharma AL (2019) J Solid State Electrochem 23(4):997–1059
Kubota K, Kumakura S, Yoda Y, Kuroki K, Komab S (2017) Adv Energy Mater. https://doi.org/10.1002/aenm.201703415 Wiley on Line
Maile NC et al (2019) J Mater Sci Mater Electron 30:5555–5566
Libich J, Máca J, Vondrák J, Čech O, MSedlaříková M (2018) J Energy Storage 17:224–227
Ma Y, Zha M, Dong Y, Li L, Hu G (2019) Mater Res Expr 6(11):6. https://doi.org/10.1088/2053-1591/ab45bd
Najib S, Erdem E (2019) Nanoscale Adv 1(8):2817–2827
Yadav HM, Ghodake GS, Kim DY, Ramesh S, Maile NC, Lee DS, Shinde SK (2019) Colloids Surf 184 184:110500
Shinde SK, Jalak MB, Kim SY, Yadav HM, Ghodake GS, Kadam AA, Kim DY (2018) Ceram Int 44(18):23102–23108
Shinde SK, Mohite SM, Kadam AA, Yadav HM, Ghodake GS, Rajpure KY, Lee DS, Kim DY (2019) J Electroanalyt Chem 850:113433
Bryan AM, Santino LM, Lu Y, Acharya S, D’Arcy JM (2016) Chem Mater 28(17):5989–5998
Hao X, Zhao J, Li Y, Zhao Y, Ma D, Li L (2011) Colloid Surf Physicochem Eng Aspect 374(1-3):42–47
Zhou T, Mo S, Zhou S, Zou W, Liu Y, Yuan D (2011) J Mater Sci 46(10):3337–3342
Zhang X, Yu P, Zhang D, Zhang H, Sun X, Ma Y (2013) Mater Lett 92:401–404
Zhang X, Sun X, Chen Y, Zhang D, Ma Y (2012) Mater Lett 68:336–339
Sobaszek M, Siuzdak K, Sawczak M, Ryl J, Bogdanowicz R (2016) Thin Solid Films 601(Supplement C):35–40
Zhou M, Glushenkov AM, Kartachova O, Li Y, Chen Y (2015) J ElectrochemSoc 162(5):A5065–A5069
Salari M, Aboutalebi SH, Chidembo AT, Nevirkovets IP, Konstantinov K, Liu HK (2012) Phys Chem Chem Phys 14(14):4770–4779
Wang T, Peng Z, Wang Y, Tang J, Zheng G (2013) Sci Rep 3:1–9
Tang H, Sui Y, Zhu X, Bao Z (2015) Nanoscale Res Lett 10(1):260–270
Yan J, Fan Z, Sun W, Ning G, Wei T, Zhang Q, Zhang R, Zhi L, Wei F (2012) Adv Funct Mater 22(12):2632–2641
Hulicova J, Puziy A, Poddubnaya O, Suarez G, Juan M, Lu G (2009) J Am Chem Soc 131:5026–5027
Futaba D, Hata K, Yamada T, Hiraoka T, Hayamizu Y, Kakudate Y, Tanaike O, Hatori H, Yumura M, Iijima S (2006) Nat Mater 12:987–994
An Z (2020) Materials 13:716. https://doi.org/10.3390/ma13030716
Sing KSW et al (1985) Pure and Appl Chem 5:603–619
Kruk M, Jaroniec M (2001) Chem Mater 13(10):3169–3183
Wickramaratne NP (2014) Ph. D. Thesis, Kent State University, USA
Guerra E, Shanmugharaj A, Choi W, Ryu S (2013) Appl Catal A 468:467–474
Sheshmani S, Fashapoyeh M (2013) J Acta Chim Slov 60:813–822
Alam S, Sharma N, Kumar L (2017) Graphene 6(01):1–18
Perez A, Saja J, Manchado A (2008) J Mater Chem 18:2221–2226
Qiu S, Kalita S (2006) Mater Sci Engin A 435-436:327–332
Guo H, Wang X, Qian Q, Wang F, Xia X (2009) ACS Nano 3(9):2653–2659
Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff R (2011) Carbon 49(9):3019–3023
Jeong HK et al (2008) J Am Chem Soc 130:1362–1366
Wu Z, Ren W, Wen L, Gao L, Zhao J, Chen Z, Zhou G, Li F, Cheng H (2010) ACS Nano 4(6):3187–3194
Petit C, Seredych M, Bandosz TJ (2009) J Mater Chem 19(48):9176–9185
Fan X, Yu C, Yang J, Ling Z, Qiu J (2014) Carbon 70:130–141
Tiekun J, Fang F, Dongsheng Y, Jianliang C, Guang S (2018) Appl Surf Sci 430:438–447
Zhang W, Liu F, Li Q, Shou Q, Cheng J, Zhang L, Nelson BJ, Zhang X (2012) Phys Chem Chem Phys 14(47):16331–16337
Oku M, Hirokawa K, Ikeda S (1975) J Electron Spectrosc 7:465473
Li Y, Qu J, Gao F, Lv S, Lin S, He C, Sun J (2015) Appl Catal B 162:268–274
Sun H, Bai Y, Cheng Y, Jin W, Xu N (2006) Ind Eng Chem Res 45(14):4971–4976
Li W, Lei L, Zhao D (2016) Nature Rev Mater 1(6):16023
Tan YH (2012) J Mater Chem 22(14):6733–6745
Li H, Wang J, Chu Q, Wang Z, Zhang F, Wang S (2009) J Power Sources 190:578–586
Selvan R, Perelshtein I, Perkas N, Gedanken A (2008) J Phys Chem C 112(6):1825–1830
He L, Zhang G, Dong Y, Zhang Z, Xue S, Jiang X (2014) Nano- Micro Lett 6:38–45
Nagamuthu S, Vijayakumar S, Muralidharan G (2013) Energy and Fuels 27(6):3508–3515
Wang Y, Li H, Xia Y (2006) Adv Mater 18:2619–2623
Du X, Guo P, Song H, Chen X (2010) Electrochim Acta 55(16):4812–4819
Farma R, Deraman M, Awitdrus I, Talib I, Omar R, Manjunatha J, Ishak M, Basri N, Dolah B (2013) Int J Electrochem Sci 8:257–273
Jiang T, Chen H, Wan H, Miao L, Zhang L (2013) Electrochim Acta 114:674–680
Farsi H, Gobal F, Barzgari Z (2013) Ionics 19(2):287–294
Shah H et al (2016) Int J Electrochem Sci 11:8155–8162
Shaik D, Rosaiah P, Hussain O (2016) Mater Proceed 3:64–73
Mei BA, Munteshari O, Lau J, Dunn B, Pilon L (2018) J Phys Chem C 122(1):194–206
Choi W, Shin HC, Kim JM, Choi JY, Yoon WS (2020) J Electrochem Sci Technol 11(1):1–13
Wang W, Yao W, Chen W, Chen D, Ma Z, Lu Z (2020) Appl Sci 10(6):1907. https://doi.org/10.3390/app10061907
Bard AJ, Faulkner LR (2001) Electrochemical Methods, 2nd edn. Wiley, New Jersey, American
Lyu J et al (2019) ChemRxiv. https://doi.org/10.26434/chemrxiv.7637030.V1
Shasha J, Tiehu L, Xiong C, Tang C, Dang A, Li H, Zhao T (2019) Nanomaterials 9:1338–1350
Sun M, Tie J, Cheng G, Lin T, Peng S, Deng F, Ye F, Yu L (2015) Mater Chem A 3:1730–1736
Mousa M, Khairy M, Shehab M (2017) J Solid State Electrochem 21(4):995–1000
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The Mn3O4/TiO2/reduced graphene oxide nanocomposite was synthesized using a hydrothermal method.
• Nanotube TiO2 and spherical Mn3O4 nanoparticles were well-dispersed on reduced graphene oxide.
• The Mn3O4/TiO2/rGO ternary nanocomposite electrode showed a much higher specific capacitance than its single individual constituents.
• An asymmetric supercapacitor (ASC) constructed using a Mn3O4–TiO2–rGO (MTrGO) as a positive electrode and G as a negative electrode works steadily under a potential window of 0–1.8 V providing a high-energy density of 31.95 Wh kg−1 at a power density of 7188 W kg−1 in 6 M KOH aqueous electrolyte.
• The ASC exhibits satisfactory cycle stability with 87% capacitance retention over 1000 cycles.
Rights and permissions
About this article
Cite this article
El-Shahat, M., Mochtar, M., Rashad, M.M. et al. Single and ternary nanocomposite electrodes of Mn3O4/TiO2/rGO for supercapacitors. J Solid State Electrochem 25, 803–819 (2021). https://doi.org/10.1007/s10008-020-04837-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04837-2