Abstract
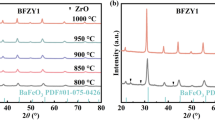
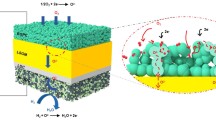
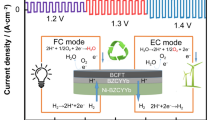
The design of new electrode materials with high redox stability has great potential for the fabrication of solid oxide fuel and electrolysis cells having a symmetrical configuration; such a configuration is particularly promising in terms of economic and technological factors due to involving a reduced number of functional materials and technological steps. Under the framework of the present study, we developed new Nd1–xBaxFe0.9M0.1O3–δ materials (where M = Cu or Ni, x = 0.4 or 0.6), characterizing their functional properties (oxygen non-stoichiometry, thermomechanical and electrical properties) under both oxidizing and reducing conditions, as well as demonstrating the principal capability of their application as symmetrical electrodes in proton-conducting electrochemical cells. The obtained results demonstrate the desirability of a low barium content due to decreased thermal expansion coefficients and chemical strain contribution and Cu-doping due to the formation of an electrochemically active scaffold having nano-sized sediments. The Nd0.6Ba0.4Fe0.9Cu0.1O3–δ electrodes fabricated onto the BaCe0.5Zr0.3Y0.1Yb0.1O3–δ proton-conducting electrolytes exhibit polarization resistances of 1.1 and 15.1 Ω cm2 at 600 °C in wet air and wet hydrogen measuring atmospheres, respectively. These reported results are among the first concerning the effective operation of symmetrical electrodes in systems with proton-conducting electrolytes.

Graphical abstract






Similar content being viewed by others
References
Burke MJ, Stephens JC (2018) Political power and renewable energy futures: a critical review. Energy Res Soc Sci 35:78–93
Buttler A, Spliethoff H (2018) Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review. Renew Sust Energ Rev 82:2440–2454
Saad W, Taleb A (2017) The causal relationship between renewable energy consumption and economic growth: evidence from Europe. Clean Techn Environ Policy 20:127–136
Hussain A, Arif SM, Aslam M (2017) Emerging renewable and sustainable energy technologies: state of the art. Renew Sust Energ Rev 71:12–28
Sreedhar I, Agarwal B, Goyal P, Singh SA (2019) Recent advances in material and performance aspects of solid oxide fuel cells. J Electroanal Chem 848:113315
Boldrin P, Brandon NP (2019) Progress and outlook for solid oxide fuel cells for transportation applications. Nat Catal 2:571–577
Venkataraman V, Pérez-Fortes M, Wang L, Hajimolana YS, Boigues-Muñoz C, Agostini A, McPhail SJ, Maréchal F, Van Herle J, Aravind PV (2019) Reversible solid oxide systems for energy and chemical applications – review & perspectives. J Energy Storage 24:100782
Shu L, Sunarso J, Hashim SS, Mao J, Zhou W, Liang F (2019) Advanced perovskite anodes for solid oxide fuel cells: a review. Int J Hydrog Energy 44:31275–31304
Ling Y, Wang X, Ma Z, Wei K, Wu Y, Khan M, Zheng K, Shen S, Wang S (2020) Review of experimental and modelling developments for ceria-based solid oxide fuel cells free from internal short circuits. J Mater Sci 55:1–23
Wang F, Lyu Y, Chu D, Jin Z, Zhang G, Wang D (2019) The electrolyte materials for SOFCs of low-intermediate temperature: review. Mater Sci Technol 35:1551–1562
Jaiswal N, Tanwar K, Suman R, Kumar D, Upadhyay S, Parkash O (2019) A brief review on ceria based solid electrolytes for solid oxide fuel cells. J Alloys Compd 781:984–1005
Zakaria Z, Hassan SH, Shaari N, Yahaya AZ, Kar YB (2020) A review on recent status and challenges of yttria stabilized zirconia modification to lowering the temperature of solid oxide fuel cells operation. Int J Energy Res 44:631–650
Meng Y, Gao J, Zhao Z, Amoroso J, Tong J, Brinkman KS (2019) Review: recent progress in low-temperature proton-conducting ceramics. J Mater Sci 54:9291–9312
Kim J, Sengodan S, Kim S, Kwon O, Bu Y, Kim G (2019) Proton conducting oxides: a review of materials and applications for renewable energy conversion and storage. Renew Sust Energ Rev 109:606–618
Medvedev D (2019) Trends in research and development of protonic ceramic electrolysis cells. Int J Hydrog Energy 44:26711–26740
Loureiro FJA, Nasani N, Reddy GS, Munirathnam NR, Fagg DP (2019) A review on sintering technology of proton conducting BaCeO3–BaZrO3 perovskite oxide materials for protonic ceramic fuel cells. J Power Sources 483:226991
Lee YH, Chang I, Cho GY, Park J, Yu W, Tanveer WH, Cha SW (2018) Thin film solid oxide fuel cells operating below 600 °C: a review. Int J Precis Eng Manuf Green Technol 5:441–453
Wang W, Medvedev D, Shao Z (2018) Gas humidification impact on the properties and performance of perovskite-type functional materials in proton-conducting solid oxide cells. Adv Funct Mater 28:1802592
Dai H, Kou H, Wang H, Bi L (2018) Electrochemical performance of protonic ceramic fuel cells with stable BaZrO3-based electrolyte: a mini-review. Electrochem Commun 96:11–15
Fan L, Zhu B, Su P-C, He C (2018) Nanomaterials and technologies for low temperature solid oxide fuel cells: recent advances, challenges and opportunities. Nano Energy 45:148–176
Rafique M, Nawaz H, Rafique MS, Tahir MB, Nabi G, Khalid NR (2018) Material and method selection for efficient solid oxide fuel cell anode: recent advancements and reviews. Int J Energy Res 43:2423–2446
Connor PA, Yue X, Savaniu CD, Price R, Triantafyllou G, Cassidy M, Kerherve G, Payne DJ, Maher RC, Cohen LF, Tomov RI, Glowacki BA, Kumar RV, Irvine JTC (2018) Tailoring SOFC electrode microstructures for improved performance. Adv Energy Mater 8:1800120
Ren R, Wang Z, Xu C, Sun W, Qiao J, Rooney DW, Sun K (2019) Tuning the defects of the triple conducting oxide BaCo0.4Fe0.4Zr0.1Y0.1O3−δ perovskite toward enhanced cathode activity of protonic ceramic fuel cells. J Mater Chem A 7:18365–18372
Song Y, Chen Y, Wang W, Zhou C, Zhong Y, Yang G, Zhou W, Liu M, Shao Z (2019) Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule 3:2842–2853
Zohourian R, Merkle R, Raimondi G, Maier J (2018) Mixed-conducting perovskites as cathode materials for protonic ceramic fuel cells: understanding the trends in proton uptake. Adv Funct Mater 28:1801241
Pikalova E, Kolchugin A, Koroleva M, Vdovin G, Farlenkov A, Medvedev D (2019) Functionality of an oxygen Ca3Co4O9+δ electrode for reversible solid oxide electrochemical cells based on proton-conducting electrolytes. J Power Sources 438:226996
Hashim SS, Liang F, Zhou W, Sunars J (2019) Cobalt-free perovskite cathodes for solid oxide fuel cells. ChemElectroChem 6:3549–3569
Lyagaeva J, Medvedev D, Pikalova E, Plaksin S, Brouzgou A, Demin A, Tsiakaras P (2017) A detailed analysis of thermal and chemical compatibility of cathode materials suitable for BaCe0.8Y0.2O3–δ and BaZr0.8Y0.2O3–δ proton electrolytes for solid oxide fuel cell application. Int J Hydrog Energy 42:1715–1723
Lyagaeva J, Danilov N, Tarutin A, Vdovin G, Medvedev D, Demin A, Tsiakaras P (2018) Designing a protonic ceramic fuel cell with novel electrochemically active oxygen electrodes based on doped Nd0.5Ba0.5FeO3−δ. Dalton Trans 47(24):8149–8157
Shi H, Ding Z, Ma G (2016) Electrochemical performance of cobalt-free Nd0.5Ba0.5Fe1–xNixO3−δ cathode materials for intermediate temperature solid oxide fuel cells. Fuel Cells 16:258–262
Bamburov AD, Markov AA, Patrakeev MV, Leonidov IA (2018) Oxygen nonstoichiometry and defect equilibria in La0.49Sr0.5–xBaxFeO3–δ. Mater Lett 213:58–61
Kundu AK, Mychinko MY, Caignaert V, Lebedev OI, Volkova NE, Deryabina KM, Cherepanov VA, Raveau B (2015) Coherent intergrowth of simple cubic and quintuple tetragonal perovskites in the system Nd2−εBa3+ε (Fe,Co)5O15−δ. J Solid State Chem 231:36–41
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767
Birks N, Meier GH, Pettit FS (2006) Introduction to the high temperature oxidation of metals, 2nd edn. Cambridge University Press, Cambridge
Zhu T, Troiani HE, Mogni LV, Han M, Barnett SA (2018) Ni-substituted Sr(Ti,Fe)O3 SOFC anodes: achieving high performance via metal alloy nanoparticle exsolution. Joule 2:478–496
Carneiro J, Nikolla E (2019) Nanoengineering of solid oxide electrochemical cell technologies: an outlook. Nano Res 12:2081–2092
Hua B, Li M, Sun Y-F, Li J-H, Luo J-L (2017) Enhancing perovskite electrocatalysis of solid oxide cells through controlled exsolution of nanoparticles. ChemSusChem 10(17):3333–3341
Zhang T, Zhao Y, Zhang X, Zhang H, Yu N, Liu T, Wang Y (2019) Thermal stability of an in situ exsolved metallic nanoparticle structured perovskite type hydrogen electrode for solid oxide cells. ACS Sustain Chem Eng 7:17834–17844
Løken A, Ricote S, Wachowski S (2018) Thermal and chemical expansion in proton ceramic electrolytes and compatible electrodes. Crystals 8:365
Bishop SR, Marrocchelli D, Chatzichristodoulou C, Perry NH, Mogensen MB, Tuller HL, Wachsman ED (2014) Chemical expansion: implications for electrochemical energy storage and conversion devices. Annu Rev Mater Res 44:205–239
Merkulov OV, Markov AA, Leonidov IA, Patrakeev MV (2019) High-temperature transport in perovskite-type Ca0.25Sr0.75Fe0.75Mo0.25O3–δ. J Solid State Electrochem 23(11):3165–3171
Markov AA, Nikitin SS, Leonidov IA, Patrakeev MV (2020) Oxygen and electron transport in Ce0.1Sr0.9FeO3–δ. Solid State Ionics 344:115131
Merkulov OV, Markov AA, Naumovich EN, Shalaeva EV, Leonidov IA, Patrakeev M (2019) Non-uniform electron conduction in weakly ordered SrFe1–xMoxO3–δ. Dalton Trans 48(14):4530–4537
Merkulov OV, Naumovich EN, Patrakeev MV, Markov AA, Shalaeva EV, Kharton VV, Tsipis EV, Waerenborgh JC, Leonidov IA, Kozhevnikov VL (2017) Defect formation, ordering, and transport in SrFe1–xSixO3–δ (x = 0.05–0.20). J Solid State Electrochem 22:727–737
He W, Fan J, Zhang H, Chen M, Sun Z, Ni M (2019) Zr doped BaFeO3–δ as a robust electrode for symmetrical solid oxide fuel cells. Int J Hydrog Energy 44:32164–32169
Gu Y, Zhang Y, Zheng Y, Chen H, Ge L, Guo L (2019) PrBaMn2O5+δ with praseodymium oxide nano-catalyst as electrode for symmetrical solid oxide fuel cells. Appl Catal B Environ 257:117868
Gou M, Ren R, Sun W, Xu C, Meng X, Wang Z, Qiao J, Sun K (2019) Nb-doped Sr2Fe1.5Mo0.5O6–δ electrode with enhanced stability and electrochemical performance for symmetrical solid oxide fuel cells. Ceram Int 45:15696–15704
Afroze S, Karim AH, Cheok Q, Eriksson S, Azad AK (2019) Latest development of double perovskite electrode materials for solid oxide fuel cells: a review. Front Energy 13:770–797
Zhang Y, Knibbe R, Sunarso J, Zhong Y, Zhou W, Shao Z, Zhu Z (2017) Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv Mater 29:1700132
Zakaria Z, Mat ZA, Hassan SHA, Kar YB (2020) A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int J Energy Res 44:594–611
The Shared Access Center at the Institute of High Temperature Electrochemistry. http://www.ihte.uran.ru/?page_id=3146
Acknowledgements
The characterization of materials was carried out at the Shared Access Centre ‘Composition of Compounds’ of the Institute of High Temperature Electrochemistry (Yekaterinburg, Russia [51]).
Funding
The major part of this work was performed according to the budgetary plans of the Institute of High Temperature Electrochemistry. Dr. D. Medvedev’ scientific activity was supported by the Council of the President of the Russian Federation (scholarship no. СП-161.2018.1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 321 kb)
Rights and permissions
About this article
Cite this article
Tarutina, L.R., Lyagaeva, J.G., Farlenkov, A.S. et al. Doped (Nd,Ba)FeO3 oxides as potential electrodes for symmetrically designed protonic ceramic electrochemical cells. J Solid State Electrochem 24, 1453–1462 (2020). https://doi.org/10.1007/s10008-020-04522-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04522-4