Abstract
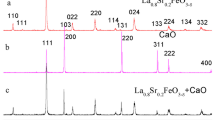
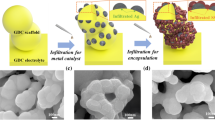
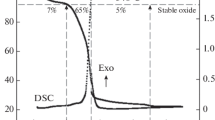
Single-phase silver (Ag)-doped La0.85-xSr0.15AgxFeO3-δ (x = 0–0.05) materials (LSAF) were synthesized by wet synthesis route and calcined at 800 °C in air. The materials exhibited no thermal degradation in Ar and synthetic air below sintering temperature at 1200 °C of the cathode for solid oxide fuel cells. Exsolution of Ag nanoparticles from the perovskite lattice at 420 °C in reducing 5% H2/N2 was investigated, and electrocatalytic activity of the cathodes towards oxygen reduction reaction for solid oxide fuel cells was demonstrated. Scanning electron microscopy confirmed exsolution of Ag nanoparticles with increased effective surface area, and the particles were distributed with a good contact on the surface of the perovskite. Electrochemical performance of novel materials was tested and compared. Enhanced cathode with Ag nanoparticles revealed the area specific resistance of 0.23 and 0.15 Ω cm2 at 800 °C in 20% O2/N2 before and after Ag exsolution, respectively. The area specific resistance of the cathode decreased with Ag exsolution, operation temperature, and increasing oxygen partial pressure.










Similar content being viewed by others
References
Sun C, Hui R, Roller J (2009) Cathode materials for solid oxide fuel cells: a review. J Solid State Electrochem 14:1125–1144
Chavan SV, Singh RN (2013) Preparation, properties, and reactivity of lanthanum strontium ferrite as an intermediate temperature SOFC cathode. J Mater Sci 48:6597–6604
Vogt UF, Sfeir J, Richter J, Soltmann C, Holtappels P (2008) B-site substituted lanthanum strontium ferrites as electrode materials for electrochemical applications. Pure Appl Chem 80:2543–2552
Hansen KK (2014) Electrochemical reduction of oxygen and nitric oxide at low temperature on La1−xSrxFeO3−δ cathodes. Electrocatalysis 5:256–261
Kim-Lohsoontorn P, Yim, HB, Bae, JM (2010) Electrochemical performance of Ni-YSZ, Ni/Ru-GDC, LSM-YSZ, LSCF and LSF electrodes for solid oxide electrolysis cells. Proceedings of the ASME 2010 8th International Fuel Cell Science, Engineering and Technology Conference FuelCell2010, June 14–16, 2010, Brooklyn, New York, USA p. 623–629
Hansen KK, Mogensen M (2008) Evaluation of LSF based SOFC cathodes using cone-shaped electrodes. ECS Trans 13:153–160
Sažinas R, Andersen KB, Simonsen SB, Holtappels P, Hansen KK (2019) Silver modified cathodes for solid oxide fuel cells. J Electrochem Soc 166:F79–F88
Zhu Y, Zhou W, Ran R, Chen Y, Shao Z, Liu M (2016) Promotion of oxygen reduction by exsolved silver nanoparticles on a perovskite scaffold for low-temperature solid oxide fuel cells. Nano Lett 16(1):512–518
Fan L, Chen M, Zhang H, Wang C, He C (2017) Pr2NiO4-Ag composite as cathode for low temperature solid oxide fuel cells: effects of silver loading methods and amounts. Int. J Hydrogen Energy 42:17544–17551
Sarikaya A, Petrovsky V, Dogan F (2012) Silver based perovskite nanocomposites as combined cathode and current collector layers for solid oxide fuel cells. J Electrochem Soc 159:F665–F669
Uhlenbruck S, Tietz F, Haanappel V, Sebold D, Buchkremer H-P, Stver D (2004) Silver incorporation into cathodes for solid oxide fuel cells operating at intermediate temperature. J Solid State Electrochem 8:923–927
Tarancón A, Burriel M, Santiso J, Skinner SJ, Kilner JA (2010) Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J Mater Chem 20:3799
Kilner JA, Burriel M (2014) Materials for intermediate-temperature solid-oxide fuel cells. Ann Rev Materi Res 44:365–393
Fan L, Zhu B, Chen M, Wang C, Raza R et al (2012) High performance transition metal oxide composite cathode for low temperature solid oxide fuel cells. J Power Sources 203:65–71
Jiang Z, Xia C, Chen F (2010) Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique. Electrochim Acta 55:3595–3605
Hua B, Li M, Sun YF, Li JH, Luo JL (2017) Enhancing perovskite electrocatalysis of solid oxide cells through controlled exsolution of nanoparticles. ChemSusChem 10(17):3333–3341
Fan L, Zhu B, Su P-C, He C (2018) Nanomaterials and technologies for low temperature solid oxide fuel cells: recent advances, challenges and opportunities. Nano Energy 45:148–176
Zhang Y, Knibbe R, Sunarso J, Zhong Y, Zhou W et al (2017) Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv Mater 1700132:1–33
Irvine JTS, Neagu D, Verbraeken MC, Chatzichristodoulou C, Graves C, Mogensen MB (2016) Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat Energy 1:15014
Myung JH, Neagu D, Miller DN, Irvine JT (2016) Switching on electrocatalytic activity in solid oxide cells. Nature 537:528–531
Connor PA, Yue X, Savaniu CD, Price R, Triantafyllou G et al (2018) Tailoring SOFC electrode microstructures for improved performance. Adv Energy Mater 8:1800120
Thommy L, Joubert O, Hamon J, Caldes M-T (2016) Impregnation versus exsolution: using metal catalysts to improve electrocatalytic properties of LSCM-based anodes operating at 600 °C. Int J Hydrog Energy 41:14207–14216
Gao Y, Lu Z, You TL, Wang J, Xie L, He J, Ciucci F (2018) Energetics of nanoparticle exsolution from perovskite oxides. J Phys Chem Lett 9(13):3772–3778
Kwon O, Sengodan S, Kim K, Kim G, Jeong HY et al (2017) Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat Commun 8:15967
Rioja-Monllor L, Bernuy-Lopez C, Fontaine M-L, Grande T, Einarsrud M-A (2019) Processing of high performance composite cathodes for protonic ceramic fuel cells by exsolution. J Mater Chem A 7:8609–8619
Yun DS, Joo JH, Yu JH, Yoon HC, Kim J-N, Yoo C-Y (2015) Electrochemical ammonia synthesis from steam and nitrogen using proton conducting yttrium doped barium zirconate electrolyte with silver, platinum, and lanthanum strontium cobalt ferrite electrocatalyst. J Power Sources 284:245–251
Kosaka F, Nakamura T, Otomo J (2017) Electrochemical ammonia synthesis using mixed protonic-electronic conducting cathodes with exsolved Ru-nanoparticles in proton conducting electrolysis cells. J Electrochem Soc 164:F1323–F1330
Liu S, Chuang KT, Luo J-L (2015) Double-layered perovskite anode with in situ exsolution of a Co–Fe alloy to cogenerate ethylene and electricity in a proton-conducting ethane fuel cell. ACS Catal 6:760–768
Neagu D, Tsekouras G, Miller DN, Menard H, Irvine JT (2013) In situ growth of nanoparticles through control of non-stoichiometry. Nat Chem 5(11):916–923
Sažinas R, Hansen KK (2019) Silver exsolution-enhanced electrical properties of lanthanum-based perovskites. J Mater Sci and Eng A 9:116–129
Rørmark L, Wiik K, Stølen S, Grande T (2002) Oxygen stoichiometry and structural properties of La1 – xAxMnO3±δ (A = Ca or Sr and 0 ≤ x ≤ 1). J MaterChem 12:1058–1067
Jørgensen MJ, Mogensen M (2001) Impedance of solid oxide fuel cell LSM/YSZ composite cathodes. J Electrochem Soc 148:A433
Koch S, Graves, C, Hansen, KV. Elchemea analytical DTU Energy2018 Available from: https://www.elchemea.com/
Kammer Hansen K, Menon M, Knudsen J, Bonanos N, Mogensen M (2010) The effect of a CGO barrier layer on the performance of LSM/YSZ SOFC cathodes. J Electrochem Soc 157:B309–B313
Dimesso L (2016) Pechini processes: an alternate approach of the sol–gel method, preparation, properties, and applications. In: Klein L, Aparicio M, Jitianu A (eds) Handbook of Sol-Gel Science and Technology. Springer, Cham, pp 1–22
Adler SB, Lane JA, Steele BCH (1996) Electrode kinetics of porous mixed-conducting oxygen electrodes. J Electrochem Soc 143:3554
Küngas R, Bidrawn F, Mahmoud E, Vohs JM, Gorte RJ (2012) Evidence of surface-reaction rate limitations in SOFC composite cathodes. Solid State Ionics 225:146–150
Takeda Y, Kanno R, Noda M, Tomida Y, Yamamoto O (1987) Cathodic polarization phenomena of perovskite oxide electrodes with stabilized zirconia. J Electrochem Soc 134:2656
Siebert E, Hammouche A, Kleitz M (1995) Impedance spectroscopy analysis of La1 − xSrxMnO3-yttria-stabilized zirconia electrode kinetics. Electrochim Acta 40:1741–1753
Robertson NL, Michaels JN (1991) Double layer capacitance of porous platinum electrodes in zirconia electrochemical cells. J Electrochem Soc 138:1494
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32:751–767
Millini R, Gagliardi MF, Piro G (1994) Structure, stoichiometry and phase purity of strontium-doped lanthanum manganite powders. J Mater Sci 29:4065–4069
Matula RA (1979) Electrical resistivity of copper, gold, palladium, and silver. J Phys Chem Ref Data 8:1147–1298
Bayraktar D, Diethelm S, Graule T, Van Herle J, Holtappels P (2008) Properties of B-site substituted La0.5Sr0.5FeO3-δ perovskites for application in oxygen separation membranes. J Electroceram 22:55–60
Moriche R, Marrero-López D, Gotor FJ, Sayagués MJ (2014) Chemical and electrical properties of LSM cathodes prepared by mechanosynthesis. J Power Sources 252:43–50
Acknowledgments
Rokas Sažinas (RS) would like to acknowledge the financial support from Villum Fonden (Vellux Foundation) for the research activities performed at DTU Energy. RS would like to acknowledge and thank Prof. Peter Holtappels for the collaboration in interpretation of the results of the manuscript. RS also acknowledges DTU Energy technicians and employees Ebtisam Abdellahi, Annelise Mikkelsen, Jens F. S. Borchsenius, and Belma Talic for the qualified technical support during the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sažinas, R., Andersen, K.B. & Hansen, K.K. Facilitating oxygen reduction by silver nanoparticles on lanthanum strontium ferrite cathode. J Solid State Electrochem 24, 609–621 (2020). https://doi.org/10.1007/s10008-020-04505-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04505-5