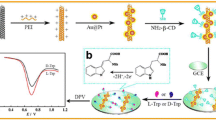
Abstract
Chirality is a universal characteristic of natural systems and discrimination of enantiomers of a chiral molecule plays a major role particularly in chemical biology and in pharmacology. In this study, a novel electrochemical chiral sensor was developed for direct discrimination of D- and L-tryptophan (Trp) in an aqueous medium. The chiral sensor was produced by hierarchical modification of reduced graphene oxide, gold nanoparticles, poly-L-cysteine, and poly-L-phenylalanine methyl ester on the glassy carbon electrode. Each of the layers was produced by electrochemical techniques, such as electrochemical reduction and polymerization. After structural and morphological characterizations, the electrochemical behaviors of the enantiomeric pairs of Trp at the modified electrode were investigated by cyclic voltammetry and square wave voltammetry. A distinctive separation between the oxidation peak potentials of D- and L-Trp was observed at 0.73 and 0.83 V, respectively. In order to investigate the amperometric response towards D- and L-Trp, chronoamperometry technique was also used in the concentration range of 0.1–0.8 mM. Furthermore, the electrochemical performance of the modified electrodes was investigated in a mixed solution of D- and L-Trp. The results showed that the prepared electrode could be used as an electrochemical chiral sensor for Trp enantiomers.

Graphical abstract










Similar content being viewed by others
References
Evans AC, Meinert C, Giri C, Goesmann F, Meierhenrich UJ (2012) Chirality, photochemistry and the detection of amino acids in interstellar ice analogues and comets. Chem Soc Rev 41(16):5447. https://doi.org/10.1039/c2cs35051c
Gopal R, Seo CH, Song PI, Park Y (2013) Effect of repetitive lysine-tryptophan motifs on the bactericidal activity of antimicrobial peptides. Amino Acids 44(2):645–660. https://doi.org/10.1007/s00726-012-1388-6
Brückner H, Becker D, Lüpke M (1993) Chirality of amino acids of microorganisms used in food biotechnology. Chirality 5(5):385–392. https://doi.org/10.1002/chir.530050521
González-Burgos I, Olvera-Cortés E, Del Angel-Meza AR, Feria-Velasco A (1995) Serotonin involvement in the spontaneous alternation ability: a behavioral study in tryptophan-restricted rats. Neurosci Lett 190(2):143–145. https://doi.org/10.1016/0304-3940(95)11519-3
Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG (2003) Tryptophan metabolites and brain disorders. Clin Chem Lab Med 41(7):852–859. https://doi.org/10.1515/CCLM.2003.129
Lam H, Oh DC, Cava F et al (2009) D-amino acids govern stationary phase cell wall remodeling in bacteria. Science (80- ) 325:1552–1555. https://doi.org/10.1126/science.1178123
Notarangelo FM, Wang XD, Horning KJ, Schwarcz R (2016) Role of d-amino acid oxidase in the production of kynurenine pathway metabolites from d-tryptophan in mice. J Neurochem 136(4):804–814. https://doi.org/10.1111/jnc.13455
Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13(7):465–477. https://doi.org/10.1038/nrn3257
Xiao Q, Lu S, Huang C, Su W, Huang S (2016) Novel N-doped carbon dots/β-cyclodextrin nanocomposites for enantioselective recognition of tryptophan enantiomers. Sensors (Basel, Switzerland) 16(11):1874. https://doi.org/10.3390/s16111874
Wei Y, Wang S, Shuang S, Dong C (2010) Chiral discrimination between d- and l-tryptophan based on the alteration of the fluorescence lifetimes by the chiral additives. Talanta 81(4-5):1800–1805. https://doi.org/10.1016/j.talanta.2010.03.044
Li H, Li F, Han C, Cui Z, Xie G, Zhang A (2010) Highly sensitive and selective tryptophan colorimetric sensor based on 4,4-bipyridine-functionalized silver nanoparticles. Sensors Actuators B Chem 145(1):194–199. https://doi.org/10.1016/j.snb.2009.11.062
Peng Z, Jiang Z, Huang X, Li Y (2016) A novel electrochemical sensor of tryptophan based on silver nanoparticles/metal–organic framework composite modified glassy carbon electrode. RSC Adv 6(17):13742–13748. https://doi.org/10.1039/C5RA25251B
Prasad BB, Madhuri R, Tiwari MP, Sharma PS (2010) Enantioselective recognition of d- and l-tryptophan by imprinted polymer-carbon composite fiber sensor. Talanta 81(1-2):187–196. https://doi.org/10.1016/j.talanta.2009.11.055
Raoof JB, Ojani R, Baghayeri M (2009) Simultaneous electrochemical determination of glutathione and tryptophan on a nano-TiO2/ferrocene carboxylic acid modified carbon paste electrode. Sensors Actuators B Chem 143(1):261–269. https://doi.org/10.1016/j.snb.2009.08.046
Li SJ, Deng DH, Pang H, Liu L, Xing Y, Liu SR (2012) Preparation of electrochemically reduced graphene oxide-modified electrode and its application for determination of p-aminophenol. J Solid State Electrochem 16(9):2883–2889. https://doi.org/10.1007/s10008-012-1720-9
Dai X, Nekraseova O, Hyde ME, Compton RG (2004) Anodic stripping voltammetry of arsenic(III) using gold nanoparticle-modified electrodes. Anal Chem 76(19):5924–5929. https://doi.org/10.1021/ac049232x
Chao M, Ma X (2015) Convenient electrochemical determination of sunset yellow and tartrazine in food samples using a poly(L-phenylalanine)-modified glassy carbon electrode. Food Anal Methods 8(1):130–138. https://doi.org/10.1007/s12161-014-9879-6
Kong Y, Zhao W, Yao S, Xu J, Wang W, Chen Z (2010) Molecularly imprinted polypyrrole prepared by electrodeposition for the selective recognition of tryptophan enantiomers. J Appl Polym Sci 115(4):1952–1957. https://doi.org/10.1002/app.31165
Lei W, Si W, Xu Y, Gu Z, Hao Q (2014) Conducting polymer composites with graphene for use in chemical sensors and biosensors. Microchim Acta 181(7-8):707–722. https://doi.org/10.1007/s00604-014-1160-6
Kang SZ, Chen H, Li X, Mu J (2010) Preparation of L-alanine ethyl ester modified multiwalled carbon nanotubes and their chiral discrimination between D- and L-tryptophan. Diam Relat Mater 19(10):1221–1224. https://doi.org/10.1016/j.diamond.2010.06.014
Zor E, Hatay Patir I, Bingol H, Ersoz M (2013) An electrochemical biosensor based on human serum albumin/graphene oxide/3-aminopropyltriethoxysilane modified ITO electrode for the enantioselective discrimination of d- and l-tryptophan. Biosens Bioelectron 42:321–325. https://doi.org/10.1016/j.bios.2012.10.068
Gou H, He J, Mo Z, Wei X, Hu R, Wang Y, Guo R (2016) A highly effective electrochemical chiral sensor of tryptophan enantiomers based on covalently functionalize reduced graphene oxide with L-lysine. J Electrochem Soc 163(7):B272–B279. https://doi.org/10.1149/2.0361607jes
Zor E, Morales-Narváez E, Alpaydin S, Bingol H, Ersoz M, Merkoçi A (2017) Graphene-based hybrid for enantioselective sensing applications. Biosens Bioelectron 87:410–416. https://doi.org/10.1016/j.bios.2016.08.074
Niu X, Mo Z, Gao H, Wang R, Li Z, Meng S, Guo R (2018) Highly selective tryptophan enantiomers electrochemical chiral sensor based on poly-lysine and functionalized multi-walled carbon nanotubes. J Solid State Electrochem 22(4):973–981. https://doi.org/10.1007/s10008-017-3832-8
Niu X, Yang X, Mo Z, Guo R, Liu N, Zhao P, Liu Z, Ouyang M (2019) Voltammetric enantiomeric differentiation of tryptophan by using multiwalled carbon nanotubes functionalized with ferrocene and β-cyclodextrin. Electrochim Acta 297:650–659. https://doi.org/10.1016/j.electacta.2018.12.041
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814. https://doi.org/10.1021/nn1006368
Guo H-L, Wang X-F, Qian Q-Y, Wang FB, Xia XH (2009) A green approach to the synthesis of graphene nanosheets. ACS Nano 3(9):2653–2659. https://doi.org/10.1021/nn900227d
Ohara I, Otsuka SI, Yugari Y, Ariyoshi S (1980) Inversion of D-tryptophan to L-tryptophan and excretory patterns in the rat and chick. J Nutr 110(4):641–648
Qu J, Wang Y, Dong Y, Zhu Z, Xing H (2015) Sensitive determination of catechol using a glassy carbon electrode modified with <scp>l</scp> -cysteine and ZnS:Ni/ZnS quantum dots. Anal Methods 7(1):260–265. https://doi.org/10.1039/C4AY02199A
Gu Y, Liu W, Chen R, Zhang L, Zhang Z (2013) β-Cyclodextrin-functionalized gold nanoparticles/poly( L-cysteine) modified glassy carbon electrode for sensitive determination of metronidazole. Electroanalysis 25(5):1209–1216. https://doi.org/10.1002/elan.201200529
Ma X, Chao M, Goicolea MA et al (2013) Electrocatalytic determination of maltol in food products by cyclic voltammetry with a poly(l-phenylalanine) modified electrode. Anal Methods 5(20):5823. https://doi.org/10.1039/c3ay41142g
Deng KQ, Hong ZJ, Li XF (2013) Direct electrochemical reduction of graphene oxide and its application to determination of l-tryptophan and l-tyrosine. Colloids Surf B: Biointerfaces 101:183–188. https://doi.org/10.1016/j.colsurfb.2012.06.007
Zhang Z, Yan J, Jin H, Yin J (2014) Tuning the reduction extent of electrochemically reduced graphene oxide electrode film to enhance its detection limit for voltammetric analysis. Electrochim Acta 139:232–237. https://doi.org/10.1016/j.electacta.2014.06.159
Raj MA, John SA (2013) Fabrication of electrochemically reduced graphene oxide films on glassy carbon electrode by self-assembly method and their electrocatalytic application. J Phys Chem C 117(8):4326–4335. https://doi.org/10.1021/jp400066z
Jeena SE, Gnanaprakasam P, Dakshinamurthy A, Selvaraju T (2015) Tuning the direct growth of Ag seeds into bimetallic Ag@Cu nanorods on surface functionalized electrochemically reduced graphene oxide: enhanced nitrite detection. RSC Adv 5(60):48236–48245. https://doi.org/10.1039/C5RA05730B
Finot MO, McDermott MT (2000) Characterization of n-alkanethiolate monolayers adsorbed to electrochemically deposited gold nanocrystals on glassy carbon electrodes. J Electroanal Chem 488(2):125–132. https://doi.org/10.1016/S0022-0728(00)00201-1
Rhieu SY, Reipa V (2015) Tuning the size of gold nanoparticles with repetitive oxidation-reduction cycles. Am J Nanomater 3:15–21. https://doi.org/10.12691/ajn-3-1-2
Hebié S, Holade Y, Servat K, et al. (2016) Electrochemical reactivity at free and supported gold nanocatalysts surface. In: Catal. Appl. Nano-Gold Catal. InTech, pp 101–130
Ferraz BRL, Leite FRF, Malagutti AR (2016) Simultaneous determination of ethionamide and pyrazinamide using poly(l-cysteine) film-modified glassy carbon electrode. Talanta 154:197–207. https://doi.org/10.1016/j.talanta.2016.03.058
Hosseini SR, Raoof JB, Ghasemi S, Gholami Z (2015) Synthesis of Pt-Cu/poly (o-anisidine) nanocomposite onto carbon paste electrode and its application for methanol oxidation. Int J Hydrog Energy 40(1):292–302. https://doi.org/10.1016/j.ijhydene.2014.10.104
Aydoğdu Tığ G (2017) Highly sensitive amperometric biosensor for determination of NADH and ethanol based on Au-Ag nanoparticles/poly(L-cysteine)/reduced graphene oxide nanocomposite. Talanta 175:382–389. https://doi.org/10.1016/j.talanta.2017.07.073
Shi Z, Xiao X, Mao D, Lu G (2014) Effects of the preparation method on the performance of the Cu/ZnO/Al2O3 catalyst for the manufacture of l-phenylalaninol with high ee selectivity from l-phenylalanine methyl ester. Catal Sci Technol 4(4):1132–1143. https://doi.org/10.1039/c3cy00937h
Du M, Yang T, Jiao K (2010) Immobilization-free direct electrochemical detection for DNA specific sequences based on electrochemically converted gold nanoparticles/graphene composite film. J Mater Chem 20(41):9253. https://doi.org/10.1039/c0jm01549k
Wang J, Bian C, Tong J, Sun J, Xia S (2012) L-Aspartic acid/L-cysteine/gold nanoparticle modified microelectrode for simultaneous detection of copper and lead. Thin Solid Films 520(21):6658–6663. https://doi.org/10.1016/j.tsf.2012.07.020
Wang L, Huang P, Bai J et al (2006) Simultaneous electrochemical determination of phenol isomers in binary mixtures at a poly (phenylalanine) modified glassy carbon electrode. Int J Electrochem Sci 1:403–413
Casero E, Parra-Alfambra AM, Petit-Domínguez MD, Pariente F, Lorenzo E, Alonso C (2012) Differentiation between graphene oxide and reduced graphene by electrochemical impedance spectroscopy (EIS). Electrochem Commun 20:63–66. https://doi.org/10.1016/j.elecom.2012.04.002
Amal Raj M, Abraham John S (2014) Graphene layer modified glassy carbon electrode for the determination of norepinephrine and theophylline in pharmaceutical formulations. Anal Methods 6(7):2181. https://doi.org/10.1039/c3ay42279h
Yang J, Deng S, Lei J, Ju H, Gunasekaran S (2011) Electrochemical synthesis of reduced graphene sheet-AuPd alloy nanoparticle composites for enzymatic biosensing. Biosens Bioelectron 29(1):159–166. https://doi.org/10.1016/j.bios.2011.08.011
Funding
This work was financially supported by the Necmettin Erbakan University, Research Foundation (BAP-141210005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 163 kb)
Rights and permissions
About this article
Cite this article
Erbilen, N., Zor, E., Saf, A.O. et al. An electrochemical chiral sensor based on electrochemically modified electrode for the enantioselective discrimination of D-/L-tryptophan. J Solid State Electrochem 23, 2695–2705 (2019). https://doi.org/10.1007/s10008-019-04370-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04370-x