Abstract
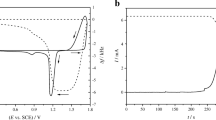
β-Lead dioxide is prepared by chemical and electrochemical routes. The chemical sample is obtained by dissolving lead tetra-acetate in distilled water at room temperature. The electrochemical sample is prepared by oxidizing cured plates in sulfuric acid with 1.05 g cm−3 specific gravity. The two powders are indexed as β-PbO2. The sample prepared by chemical route presents smaller crystallite size. When cycling the two powders up to 100 cycles between 0.5 and 1.5 V versus Hg/Hg2SO4 reference electrode, the electrochemical sample presents higher values of anodic and cathodic peak current densities and higher discharge capacity. Thermal analysis and electrochemical techniques are used to explain this difference in activity between the two samples.








Similar content being viewed by others
References
Rüetschi P, Cahan BD (1957) Anodic corrosion and hydrogen and oxygen overvoltage on lead and lead antimony alloys. J Electrochem Soc 104(7):406–413. https://doi.org/10.1149/1.2428614
Burbank J (1957) Anodization of lead and lead alloys in sulfuric acid. J Electrochem Soc 104(12):693–701. https://doi.org/10.1149/1.2428455
Rüetschi P (1963) Stability and reactivity of lead oxides. Electrochim Acta 8(5):333–342. https://doi.org/10.1016/0013-4686(63)80063-8
Bagshaw NE, Clarke RL, Halliwell B (1966) The preparation of lead dioxide for X-ray diffraction studies. J Appl Chem 16:180–184
Duisman JA, Giaugue WF (1968) Thermodynamics of the lead storage cell. The heat capacity and entropy of lead dioxide from 15 to 318.degree K. J Phys Chem 72(2):562–573. https://doi.org/10.1021/j100848a030
Caulder SM, Simon AC (1974) Thermal decomposition mechanism of formed and cycled lead dioxide electrodes and its relationship to capacity loss and battery failure. J Electrochem Soc 121:1546–1551
Turner AD, Moseley PT, Hutchison JL (1984) Utilization of active material in PbO2 electrodes. Proc Electrochem Soc 84-14:267–276
Taylor EJ, Shia GA, Peters DT (1984) A precharged positive plate for lead-acid automotive battery: I. Positive plate allowing direct incorporation of PbO2. J Electrochem Soc 131(3):483–487. https://doi.org/10.1149/1.2115613
Taylor EJ, Shia GA, Peters DT (1984) A precharged positive plate for lead-acid automotive battery: II. Effects of various PbO2 types and paste formulations on precharged positive plate performance. J Electrochem Soc 131(3):487–491. https://doi.org/10.1149/1.2115614
Moseley PT, Bridger NJ (1984) Lead-acid battery cathodes incorporating chemically prepared PbO2. J Electrochem Soc 131(3):608–610. https://doi.org/10.1149/1.2115634
Hill RJ, Jessel AM (1987) The electrochemical activity of PbO2. A nuclear magnetic resonance study of hydrogen in battery and chemically prepared material. J Electrochem Soc 134(6):1326–1330. https://doi.org/10.1149/1.2100667
Tokunaga A, Tsubota M, Yonezu K, Ando K (1987) Effect of anisotropic graphite on discharge performance of positive plates in pasted-type lead-acid batteries. J Electrochem Soc 134(3):525–529. https://doi.org/10.1149/1.2100503
Rüetschi P (1992) Influence of crystal structure and interparticle contact on the capacity of PbO2 electrodes. J Electrochem Soc 139(5):1347–1351. https://doi.org/10.1149/1.2069410
Pavlov D (1992) The lead-acid battery lead dioxide active mass: a gel-crystal system with proton and electron conductivity. J Electrochem Soc 139(11):3075–3080. https://doi.org/10.1149/1.2069034
Pavlov D, Balkanov I, Halachev T, Rachev P (1989) Hydration and amorphization of active mass PbO2 particles and their influence on the electrical properties of the lead-acid battery positive plate. J Electrochem Soc 136(11):3189–3197. https://doi.org/10.1149/1.2096424
Monahov B, Pavlov D (1993) Hydrated structures in the anodic layer formed on lead electrodes in H2SO4 solution. J Appl Electrochem 23:1244–1250
Fitas R, Chelali N, Zerroual L, Djellouli B (2000) Mechanism of the reduction of α- and β-PbO2 electrodes using an all-solid-state system. Solid State Ionics 127(1–2):49–54. https://doi.org/10.1016/S0167-2738(99)00266-0
Hill RJ, Houchin MR (1985) Incorporation of hydrogen in lead dioxide by a surface hydrolysis mechanism. Electrochim Acta 30(4):559–561. https://doi.org/10.1016/0013-4686(85)80047-5
Fitas R, Zerroual L, Chelali N, Djellouli B (1996) Heat treatment of α- and β-battery lead dioxide and its relationship to capacity loss. J Power Sources 58(2):225–229. https://doi.org/10.1016/S0378-7753(96)02372-5
Fitas R, Zerroual L, Chelali N, Djellouli B (1997) Role of hydration water in the reduction process of PbO2 in lead/acid cells. J Power Sources 64(1-2):57–60. https://doi.org/10.1016/S0378-7753(96)02502-5
Fitas R, Zerroual L, Chelali N, Djellouli B (2000) Thermal degradation of α- and β-PbO2 and its relationship to capacity loss. J Power Sources 85(1):56–58. https://doi.org/10.1016/S0378-7753(99)00382-1
Zerroual L, Fitas R, Djellouli B, Chelali N (2006) Relationship between water departure and capacity loss of α and β-PbO2 using an all solid-state system: estimation of proton diffusion coefficient. J Power Sources 158(2):837–840. https://doi.org/10.1016/j.jpowsour.2005.11.011
Morales J, Petkova G, Cruz M, Caballero A (2006) Synthesis and characterization of lead dioxide active material for lead-acid batteries. J Power Sources 158:831–836
Yang S, Li R, Cai X, Xue K, Yang B, Hu X, Dai C (2017) Influence of hydrated PbO2 content on the cycling performance of lead-acid batteries. J Electrochem Soc 164(9):A2007–A2011. https://doi.org/10.1149/2.1261709jes
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Derafa, I., Zerroual, L. & Matrakova, M. On the electrochemical activity of β-lead dioxide in sulfuric acid solution: a comparative study between the chemical and electrochemical routes. J Solid State Electrochem 22, 1175–1183 (2018). https://doi.org/10.1007/s10008-017-3861-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3861-3