Abstract
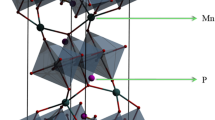
Electrochemical reactions in the particulate crystal of olivine cathodes topotactically proceed in the lithiated and delithiated phases. Optimized-synthesis conditions for 4 V class LiMn0.8Fe0.2PO4 cathode materials permitted the surface modification and cation-mixing reduction for the resulting powder particles. The discharge capacities of a Li/LiMn0.8Fe0.2PO4 cell in the C–rate range of 0.1C (17.1 mA/g) and 5C (855 mA/g) were of practical values and compatible with those of a Li/LiFePO4 cell. However, since the crystallite size of LiMn0.8Fe0.2PO4 was about 80 nm and almost half as large as LiFePO4, the electrode density of LiMn0.8Fe0.2PO4 was 83 percentage of LiFePO4, meaning the reduction of the volumetric energy density. To examine the necessity of the reduced crystallite size to obtain the excellent gravimetric energy density, the charge–density distributions around the diffusion pathways of Li+ ions for the lithiated and delithiated materials have been investigated, which allows us to discuss the atomic scale diffusivities of Li+ ions.

ᅟ






Similar content being viewed by others
References
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Andre D, Kim SJ, Lamp P, Lux SF, Maglia F, Paschosa O, Stiaszny B (2015) Future generations of cathode materials: an automotive industry perspective. J Mater Chem A 3:6709–6732
Goodenough JB, Manthiram A (2014) A perspective on electrical energy storage. MRS Communications 4:135–142
Nazri GA, Pistoia G (2003) Lithium batteries: science and technology. Kluwer Academic Publishers, Massachusetts
Scrosati B, Garche J (2010) Lithium batteries: Status, prospects and future. J Power Sources 195:2419–2430
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195:939–954
Hausbrand R, Cherkashinin G, Ehrenberg H, Gröting M, Albe K, Hess C, Jaegermann W (2015) Fundamental degradation mechanisms of layered oxide Li–ion battery cathode materials: methodology, insights and novel approaches. Mater Sci Eng B 192:3–25
Wang J, Sun X (2012) Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium–ion batteries. Energy Environ Sci 5:5163–5185
Franco AA (2013) Multiscale modelling and numerical simulation of rechargeable lithium ion batteries: concepts, methods and challenges. RSC Adv 3:13027–13058
Masquelier C, Croguennec L (2013) Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem Rev 113:6552–6591
Li D, Zhou H (2014) Two–phase transition of Li–intercalation compounds in Li–ion batteries. Mater Today 17:451–463
Dominko R, Bele M, Gaberscek M, Remskar M, Hanzel D, Pejovnik S, Jamnik J (2005) Impact of the carbon coating thickness on the electrochemical performance of LiFePO4 / C composites. J Electrochem Soc 152:A607–A610
Chang ZR, Lv HJ, Tang HW, Li HJ, Yuan XZ, Wang H (2009) Synthesis and characterization of high–density LiFePO4 /C composites as cathode materials for lithium–ion batteries. Electrochim Acta 54:4595–4599
Kang B, Ceder G (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193
Morgan D, Van der Ven A, Ceder G (2004) Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni ) olivine materials. Electrochem Solid-State Lett 7:A30–A32
Islam MS, Driscoll DJ, Fisher CAJ, Slater PR (2005) Atomic–scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine–type battery material. Chem Mater 17:5085–5092
Yang J, Tse JS (2011) Li ion diffusion mechanisms in LiFePO4: an ab initio molecular dynamics study. J Phys Chem A 115:13045–13049
Nishimura S, Kobayashi G, Ohoyama K, Kanno R, Yashima M, Yamada A (2008) Experimental visualization of lithium diffusion in LixFePO4. Nat Mater 7:707–711
Tealdi C, Spreafico C, Mustarelli P (2012) Lithium diffusion in Li1-xFePO4: the effect of cationic disorder. J Mater Chem A 22:24870–24876
Adams S (2010) Lithium ion pathways in LiFePO4 and related olivines. J Solid State Electrochem 14:1787–1792
Avdeev M, Sale M, Adams S, Rao RP (2012) Screening of the alkali–metal ion containing materials from the Inorganic Crystal Structure Database (ICSD) for high ionic conductivity pathways using the bond valence method. Solid State Ionics 225:43–46
Malik R, Abdellahi A, Ceder G (2013) A critical review of the Li insertion mechanisms in LiFePO4 electrodes. J Electrochem Soc 160:A3179–A3197
Delmas C, Maccario M, Croguennec L, Le Cras F, Weill F (2008) Lithium deintercalation in LiFePO4 nanoparticles via a domino–cascade model. Nat Mater 7:665–671
Brunetti G, Robert D, Bayle-Guillemaud P, Rouvière JL, Rauch EF, Martin JF, Colin JF, Bertin F, Cayron C (2011) Confirmation of the domino–cascade model by LiFePO4/FePO4 precession electron diffraction. Chem Mater 23:4515–4524
Dreyer W, Jamnik J, Guhlke C, Huth R, Moškon J, Gaberšček M (2010) The thermodynamic origin of hysteresis in insertion batteries. Nat Mater 9:448–453
Malik R, Zhou F, Ceder G (2011) Kinetics of non–equilibrium lithium incorporation in LiFePO4. Nat Mater 10:587–590
Bai P, Cogswell DA, Bazant MZ (2011) Suppression of phase separation in LiFePO4 nanoparticles during battery discharge. Nano Lett 11:4890–4896
Orikasa Y, Maeda T, Koyama Y, Murayama H, Fukuda K, Tanida H, Arai H, Matsubara E, Uchimoto Y, Ogumi Z (2013) Direct observation of a metastable crystal phase of LixFePO4 under electrochemical phase transition. J Am Chem Soc 135:5497–5500
Liu Q, He H, Li ZF, Liu Y, Ren Y, Lu W, Lu J, Stach EA, Xie J (2014) Rate–dependent, Li–ion insertion/deinsertion behavior of LiFePO4 cathodes in commercial 18650 LiFePO4 cells. Appl Mater Interfaces 6:3282–3289
Suo L, Han W, Lu X, Gu L, Hu YS, Li H, Chen D, Chen L, Tsukimoto S, Ikuhara Y (2012) Highly ordered staging structural interface between LiFePO4 and FePO4. Phys Chem Chem Phys 14:5363–5367
Bakenov Z, Taniguchi I (2010) Physical and electrochemical properties of LiMnPO4/C composite cathode prepared with different conductive carbons. J Power Sources 195:7445–7451
Oh SM, Myung ST, Park JB, Scrosati B, Amine K, Sun YK (2012) Double–structured LiMn0.85Fe0.15PO4 coordinated with LiFePO4 for rechargeable lithium batteries. Angew Chem Int Ed 51:1853–1856
Aravindan V, Gnanaraj J, Lee YS, Madhavi S (2013) LiMnPO4 – a next generation cathode material for lithium–ion batteries. J Mater Chem A 1:3518–3539
Yamada A, Kudo Y, Liu KY (2001) Reaction mechanism of the olivine–type Lix ( Mn0.6Fe0.4) PO4 ( 0 ⩽ x ⩽ 1 ). J Electrochem Soc 148:A747–A754
Meethong N, Huang HYS, Speakman SA, Carter WC, Chiang YM (2007) Strain accommodation during phase transformations in olivine–based cathodes as a materials selection criterion for high–power rechargeable batteries. Adv Funct Mater 17:1115–1123
Yashima M (2008) Crystal structures, structural disorders and diffusion paths of ionic conductors from diffraction experiments. Solid State Ionics 179:797–803
Brown ID (2009) Recent developments in the methods and applications of the bond valence model. Chem Rev 109:6858–6919
Filsø MØ, Turner MJ, Gibbs GV, Adams S, Spackman MA, Iversen BB (2013) Visualizing lithium–ion migration pathways in battery materials. Chem Eur J 19:15535–15544
Han J, Zhu J, Li Y, Yu X, Wang S, Wu G, Xie H, Vogel SC, Izumi F, Momma K, Kawamura Y, Huang Y, Goodenough JB, Zhao Y (2012) Experimental visualization of lithium conduction pathways in garnet–type Li7La3Zr2O12. Chem Commun 48:9840–9842
Anurova NA, Blatov VA, Ilyushin GD, Blatova OA, Ivanov-Shits AK, Dem’yanets LN (2009) Analysis of Li+ cation migration paths in oxygen–containing compounds. Russian J Electrochem 45:417–428
Kuroiwa Y, Aoyagi S, Sawada A, Harada J, Nishibori E, Takata M, Sakata M (2011) Evidence for Pb–O covalency in tetragonal PbTiO3. Phys Rev Lett 87:217601
Tanaka H, Takata M, Nishibori E, Kato K, Iishi T, Sakata M (2002) ENIGMA: maximum–entropy method program package for huge systems. J Appl Crystallogr 35:282–286
Nishibori E, Sunaoshi E, Yoshida A, Aoyagi S, Kato K, Takata M, Sakata M (2007) Accurate structure factors and experimental charge densities from synchrotron X–ray powder diffraction data at SPring–8. Acta Cryst A63:43–52
Takata M (2008) The MEM/Rietveld method with nano–applications – accurate charge–density studies of nano–structured materials by synchrotron–radiation powder diffraction. Acta Cryst A64:232–245
Nishibori E, Shibata T, Kobayashi W, Morimoto Y (2015) Bonding nature of LiCoO2 by topological analysis of electron density from X–ray diffraction. Electrochemistry 83:840–842
Mishima Y, Hojo T, Nishio T, Sadamura H, Oyama N, Moriyoshi C, Kuroiwa Y (2013) MEM charge density study of olivine LiMPO4 and MPO4 (M = Mn, Fe) as cathode materials for lithium–ion batteries. J Phys Chem C 117:2608–2615
Mishima Y, Hojo T, Nishio T, Kajiyama A, Moriyoshi C, Kuroiwa Y (2015) Structural and electrochemical properties of 20–micron Li(Co1-xLix)O2-δ (x > 0) agglomerates with layered structures: identification of tetravalent cobalt. J Phys Chem Solids 87:48–52
Mishima Y, Honda S, Sadamura H, Nakayama N, Moriyoshi C, Kuroiwa Y (2011) Characterization of carbon composite LiMn1-xFexPO4 cathodes. IOP Conf Series: Materials Science and Engineering 18:122002
Sakata M, Sato M (1990) Accurate structure analysis by the maximum–entropy method. Acta Cryst A46:263–270
Momma K, Izumi F (2011) VESTA 3 for three–dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Chen J, Vacchio MJ, Wang S, Chernova N, Zavalij PY, Whittingham MS (2008) The hydrothermal synthesis and characterization of olivines and related compounds for electrochemical applications. Solid State Ionics 178:1676–1693
Mishima Y, Nishio T, Hojo T, Sadamura H (2014) Method for producing carbon composite lithium manganese iron phosphate particle powder, carbon composite lithium manganese iron phosphate particle powder, and nonaqueous electrolyte secondary battery using carbon composite lithium manganese iron phosphate particle powder. WO2014034775 A1
Malik R, Burch D, Bazant M, Ceder G (2010) Particle size dependence of the ionic diffusivity. Nano Lett 10:4123–4127
Seo DH, Gwon H, Kim SW, Kim J, Kang K (2010) Multicomponent olivine cathode for lithium rechargeable batteries: a first–principles study. Chem Mater 22:518–523
Acknowledgments
The authors would like to thank Mr. Takuma Hojo, Mr. Taisei Inoue, and Mr. Takahisa Nishio for their help and support. The Synchrotron XRD experiments were performed at SPring–8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (proposal nos. 2009B0084, 2010A0084, and 2010B0084).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishima, Y., Moriyoshi, C. & Kuroiwa, Y. Electrochemical and structural study on LiMn0.8Fe0.2PO4 and Mn0.8Fe0.2PO4 battery cathodes: diffusion limited lithium transport. J Solid State Electrochem 21, 3221–3228 (2017). https://doi.org/10.1007/s10008-017-3636-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3636-x