Abstract
Context
Acinetobacter baumannii, one of the critical ESKAPE pathogens, is a highly resilient, multi-drug-resistant, Gramnegative, rod-shaped, highly pathogenic bacteria. It is responsible for almost 1–2% of all hospital-borne infections in immunocompromised patients and causes community outbreaks. Because of its resilience and MDR characteristics, looking for new strategies to check the infections related to this pathogen becomes paramount. The enzymes involved in the peptidoglycan biosynthetic pathway are attractive and the most promising drug targets. They contribute to the formation of the bacterial envelope and help to maintain the rigidity and integrity of the cell. The MurI (glutamate racemase) is one of the crucial enzymes that aid in the formation of the pentapeptide responsible for the interlinkage of peptidoglycan chains. It converts l-glutamate to d-glutamate, which is required to synthesise the pentapeptide chain.
Methods
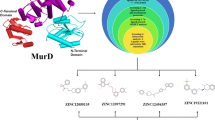
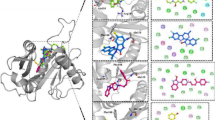
In this study, the MurI protein of A. baumannii (strain AYE) was modelled and subjected to high-throughput virtual screening against the enamine-HTSC library, taking UDP-MurNAc-Ala binding site as the targeted site. Four ligand molecules, Z1156941329 (N-(1-methyl-2-oxo-3,4-dihydroquinolin-6-yl)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxamide), Z1726360919 (1-[2-[3-(benzimidazol-1-ylmethyl)piperidin-1-yl]-2-oxo-1-phenylethyl]piperidin-2-one), Z1920314754 (N-[[3-(3-methylphenyl)phenyl]methyl]-8-oxo-2,7-diazaspiro[4.4]nonane-2-carboxamide) and Z3240755352 (4R)-4-(2,5-difluorophenyl)-1-(4-fluorophenyl)-1,3a,4,5,7,7a-hexahydro-6H-pyrazolo[3,4-b]pyridin-6-one), were identified to be the lead candidates based on Lipinski’s rule of five, toxicity, ADME properties, estimated binding affinity and intermolecular interactions. The complexes of these ligands with the protein molecule were then subjected to MD simulations to scrutinise their dynamic behaviour, structural stability and effects on protein dynamics. The molecular mechanics/Poisson–Boltzmann surface area–based binding free energy analysis was also performed to compute the binding free energy of protein-ligand complexes, which offered the following values −23.32 ± 3.04 kcal/mol, −20.67 ± 2.91kcal/mol, −8.93 ± 2.90 kcal/mol and −26.73 ± 2.95 kcal/mol for MurI-Z1726360919, MurI-Z1156941329, MurI-Z3240755352 and MurI-Z3240755354 complexes respectively. Together, the results from various computational analyses utilised in this study proposed that Z1726360919, Z1920314754 and Z3240755352 could act as potential lead molecules to suppress the function of MurI protein from Acinetobacter baumannii.








Similar content being viewed by others
Data availability
Data and materials are available on request from the authors.
Abbreviations
- ADME:
-
Absorption, Distribution, Metabolism and Excretion
- DCCM:
-
Dynamical cross-correlation matrix
- FEL:
-
Free energy landscape
- MD:
-
Molecular dynamics
- MM/PBSA:
-
Molecular mechanics/Poisson–Boltzmann surface area
- ESKAPE:
-
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp
- MDR:
-
Multi-drug resistant
- XDR:
-
Extensive drug-resistant
- UDP-MurNAc-Ala:
-
Uridine diphosphate N-acetylmuramylalanine
References
De Oliveira DMP et al (2020) Antimicrobial Resistance in ESKAPE Pathogens. Clin Microbiol Rev 33(3)
Pendleton JN, Gorman SP, Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11(3):297–308
Mulani MS et al (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10:539
Howard A et al (2012) Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3(3):243–250
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21(3):538–582
Maragakis LL, Perl TM (2008) Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46(8):1254–1263
Antunes LC, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71(3):292–301
Dijkshoorn L, Nemec A, Seifert H (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5(12):939–951
Smith CA (2006) Structure, function and dynamics in the mur family of bacterial cell wall ligases. J Mol Biol 362(4):640–655
Neshani A et al (2020) Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections. Microb Pathog 146:104238
McConnell MJ, Pachon J (2010) Active and passive immunization against Acinetobacter baumannii using an inactivated whole cell vaccine. Vaccine 29(1):1–5
Garcia-Quintanilla M, Pulido MR, McConnell MJ (2013) First steps towards a vaccine against Acinetobacter baumannii. Curr Pharm Biotechnol 14(10):897–902
Kaur H, Kalia M, Taneja N (2021) Identification of novel non-homologous drug targets against Acinetobacter baumannii using subtractive genomics and comparative metabolic pathway analysis. Microb Pathog 152:104608
Kaur N et al (2013) Identification of druggable targets for Acinetobacter baumannii via subtractive genomics and plausible inhibitors for MurA and MurB. Appl Biochem Biotechnol 171(2):417–436
Egan AJF, Errington J, Vollmer W (2020) Regulation of peptidoglycan synthesis and remodelling. Nat Rev Microbiol 18(8):446–460
Le NH et al (2020) Peptidoglycan editing provides immunity to Acinetobacter baumannii during bacterial warfare. Sci Adv 6(30):eabb5614
Lundqvist T et al (2007) Exploitation of structural and regulatory diversity in glutamate racemases. Nature 447(7146):817–822
de Jonge BL et al (2009) Pyrazolopyrimidinediones are selective agents for Helicobacter pylori that suppress growth through inhibition of glutamate racemase (MurI). Antimicrob Agents Chemother 53(8):3331–3336
Doublet P et al (1993) The murI gene of Escherichia coli is an essential gene that encodes a glutamate racemase activity. J Bacteriol 175(10):2970–2979
The UniProt C (2017) UniProt: the universal protein knowledgebase. Nucleic Acids Res 45(D1):D158–D169
Bernstein FC et al (1977) The Protein Data Bank. A computer-based archival file for macromolecular structures. Eur J Biochem 80(2):319–324
Johnson M et al (2008) NCBI BLAST: a better web interface. Nucleic Acids Res 36(Web Server issue):W5–W9
Waterhouse A et al (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303
Heo L, Park H, Seok C (2013) GalaxyRefine: protein structure refinement driven by side-chain repacking. Nucleic Acids Res 41(Web Server issue):W384–W388
Morris AL et al (1992) Stereochemical quality of protein structure coordinates. Proteins 12(4):345–364
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2(9):1511–1519
Bowie JU, Luthy R, Eisenberg D (1991) A method to identify protein sequences that fold into a known three-dimensional structure. Science 253(5016):164–170
Luthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356(6364):83–85
Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. Methods Mol Biol 1263:243–250
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Sander T et al (2015) DataWarrior: an open-source program for chemistry aware data visualization and analysis. J Chem Inf Model 55(2):460–473
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717
Morris GM et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Van Der Spoel D et al (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718
Schuttelkopf AW, van Aalten DM (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 60(Pt 8):1355–1363
Huang W, Lin Z, van Gunsteren WF (2011) Validation of the GROMOS 54A7 force field with respect to beta-peptide folding. J Chem Theory Comput 7(5):1237–1243
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10(5):449–461
Laskowski RA et al (2018) PDBsum: structural summaries of PDB entries. Protein Sci 27(1):129–134
O'Boyle NM et al (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33
Dahlin JL et al (2015) PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J Med Chem 58(5):2091–2113
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22(12):2577–2637
Acknowledgements
Dr. Amit Kumar Singh thanks Indian Council of Medical Research (ICMR) and Indian National Science Academy (INSA), New Delhi, India. The authors thank Sharda University for the support.
Author information
Authors and Affiliations
Contributions
Ankit Kumar: data curation; formal analysis; methodology; software; visualisation; writing—original draft; writing—review and editing. Ekampreet Singh: formal analysis; methodology; software; visualisation; writing-review and editing; Rajat Kumar Jha: formal analysis, writing-review and editing; Rameez Jabeer Khan: formal analysis; Monika Jain: formal analysis, writing—review and editing. Sudeep Varshney: software, writing—review and editing. Jayaraman Muthukumaran: validation, writing—review and editing. Amit Kumar Singh: conceptualisation, investigation, supervision, validation, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 15 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Singh, E., Jha, R.K. et al. Targeting multi-drug-resistant Acinetobacter baumannii: a structure-based approach to identify the promising lead candidates against glutamate racemase. J Mol Model 29, 188 (2023). https://doi.org/10.1007/s00894-023-05587-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05587-4