Abstract
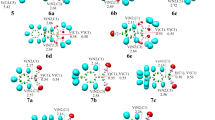
A computational investigation of the aerobic oxidative C–C bond cleavage reaction of glycol catalyzed by an Anderson-type heteropolyanion HPA [IMo6O24]5− in the presence of acetonitrile as solvent has been performed at the WB97XD/6-31G(d,p)/lanl2dz level. Two reaction pathways have been identified. The catalytic cycle of each pathway consists of three steps: oxidation cleavage of a glycol molecule by the HPA, oxidation of the HPA by one dioxygen molecule, and, finally, oxidation of a second glycol and regeneration of the catalyst. These reaction pathways have been thoroughly investigated in terms of energetic, natural bond orbital (NBO), natural charges, and geometrical parameters. It is found that (i) even though the top oxygen atoms of the Anderson heteropolyanion are not the most negatively charged ones, they are more likely to react with the diol hydroxyl groups, (ii) a direct relationship between the presence of the iodine ion I(VII) and the studied oxidation reaction could not be identified, and (iii) in terms of energy, the transfer of the two hydrogen atoms is the most energetic step.
Graphical abstract








Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Anastas P, Bartlett L, Kirchhoff M, Williamson T (2000) The role of catalysis in the design, development, and implementation of green chemistry. Catal Today 55:11–22. https://doi.org/10.1016/S0920-5861(99)00222-9
Rodríguez-Padrón D, Puente-Santiago A, Balu A, Muñoz-Batista M, Luque R (2018) ChemCatChem 11:18–38. https://doi.org/10.1002/cctc.201801248
Delidovich I, Palkovits R (2016) Green Chem 18:590–593. https://doi.org/10.1039/C5GC90070K
Trost BM, Fleming I (eds) (1991) Comprehensive organic synthesis: selectivity, strategy, and efficiency in modern organic chemistry (vol. 8). Elsevier
Zakzeski J, Bruijnincx P, Jongerius A, Weckhuysen B (2010) The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem Rev 110:3552–3599. https://doi.org/10.1021/cr900354u
Perlin, A.: Advances in Carbohydrate Chemistry and Biochemistry Volume 60. 183–250 (2006). https://doi.org/10.1016/S0065-2318(06)60005-X
Malaprade L (1928) Bull Soc Chim Fr 43:683
Sudalai A, Khenkin A, Neumann R (2015) Org Biomol Chem 13:4374–4394. https://doi.org/10.1039/C5OB00238A
Criegee R (1931) Ber 64:260–266
Baer E, Grosheintz J, Fischer H (1939) Oxidation of 1,2-Glycols or 1,2,3-Polyalcohols by Means of Lead Tetraacetate in Aqueous Solution. J Am Chem Soc 61:2607–2609. https://doi.org/10.1021/ja01265a010
Zhou Z, Liu M, Lv L, Li C (2018) Anastas. Angew Chem 57:2616–2620. https://doi.org/10.1002/anie.201711531
Escande V, Lam C, Coish P, Anastas P (2017) Anastas. Angew Chem 56:9561–9565
Kim S, Kim D, Yang J (2014) Org Lett 16:2876–2879. https://doi.org/10.1039/D2QO00221C
El Aakel, L., Launay, F., Atlamsani, A.: Brégeault, Efficient and selective catalytic oxidative cleavage of α-hydroxy ketones using vanadium-based HPA and dioxygen. J. Chem. Commun. 2218 (2001). https://doi.org/10.1039/B106969A
Amadio E, González-Fabra J, Carraro D, Denis W, Gjoka B, Zonta C, Bartik K, Cavani F, Solmi S, Bo C et al (2018) Efficient Vanadium-Catalyzed Aerobic C−C Bond Oxidative Cleavage of Vicinal Diols. Adv Synth Catal 360:3286–3296. https://doi.org/10.1002/adsc.201800050
Shimizu M, Orita H, Suzuki K, Hayakawa T, Hamakawa S, Takehira K (1996) J Mol Catal A: Chem 114:217–220. https://doi.org/10.1016/S1381-1169(96)00320-2
Yu H, Wang J, Wu Z, Zhao Q, Dan D, Han S, Tang J, Wei Y (2019) Green Chem 21:4550–4554. https://doi.org/10.1039/C9GC02053E
Heravi M, VazinFard M, Faghihi Z (2013) Heteropoly acids-catalyzed organic reactions in water: doubly green reactions. Green Chem Lett Rev 6:282–300. https://doi.org/10.1080/17518253.2013.846415
Sun L, Su T, Li P, Xu J, Chen N, Liao W, Deng C, Ren W, Lü H (2019) Extraction Coupled with Aerobic Oxidative Desulfurization of Model Diesel Using a B-type Anderson Polyoxometalate Catalyst in Ionic Liquids. Catal Lett 149:1888–1893. https://doi.org/10.1007/s10562-019-02791-x
Wei Z, Wang J, Yu H, Han S, Wei Y (2022) Extraction Coupled with Aerobic Oxidative Desulfurization of Model Diesel Using a B-type Anderson Polyoxometalate Catalyst in Ionic Liquids. Molecules 27:5212. https://doi.org/10.3390/molecules27165212
Yu W, Zhang Y, Han Y, Li B, Shao S, Zhang L, Xie H, Yan J (2021) Microwave-Assisted Synthesis of Tris-Anderson Polyoxometalates for Facile CO 2 Cycloaddition. Inorg Chem 60:3980–3987. https://doi.org/10.1021/acs.inorgchem.1c00019
Guermi, I.N.E.H., Saal, A.: Struct. Chem. 1–10 (2022). https://doi.org/10.1007/s11224-022-02088-7
Wei Z, Ru S, Zhao Q, Yu H, Zhang G, Wei Y (2019) Highly efficient and practical aerobic oxidation of alcohols by inorganic-ligand supported copper catalysis. Green Chem 21:4069–4075. https://doi.org/10.1039/C9GC01248F
Zhou Z, Dai G, Ru S, Yu H, Wei Y (2019) Highly selective and efficient olefin epoxidation with pure inorganic-ligand supported iron catalysts. Dalton Trans. 48:14201–14205. https://doi.org/10.1039/C9DT02997D
Yu, H., Wu, Z., Wei, Z., Zhai, Y., Ru, S., Zhao, Q., Wang, J., Han, S., Wei, Y.: Commun. Chem. 2 (2019). https://doi.org/10.1038/s42004-019-0109-4
Wu Z, Zhai Y, Zhao W, Wei Z, Yu H, Han S, Wei Y (2020) An efficient way for the N formylation of amines by inorganic-ligand supported iron catalysis. Green Chem 22:737–741. https://doi.org/10.1039/C9GC03564H
Jin, P., Wei, H., Zhou, L., Wei, D., Wen, Y., Zhao, B., Wang, X., Li, B.: Mol. Catal. 510, 111705 (2021). https://doi.org/10.1016/j.mcat.2021.111705
Khenkin A, Neumann R (2002) Aerobic Oxidation of Vicinal Diols Catalyzed by an Anderson-Type Polyoxometalate, [IMo6O24]5. Adv Synth Catal 344:1017–1021. https://doi.org/10.1002/1615-4169(200210)344:9%3c1017::AID-ADSC1017%3e3.0.CO;2-X
Yu H, Zhai Y, Dai G, Ru S, Han S, Wei Y (2017) Transition-Metal-Controlled Inorganic Ligand-Supported Non-Precious Metal Catalysts for the Aerobic Oxidation of Amines to Imines. Eur J chem 23:13883–13887. https://doi.org/10.1002/chem.201703185
Zhang M, Zhai Y, Ru S, Zang D, Han S, Yu H, Wei Y (2018) Highly practical and efficient preparation of aldehydes and ketones from aerobic oxidation of alcohols with an inorganic-ligand supported iodine catalyst. Chem Comm 54:10164–10167. https://doi.org/10.1039/C8CC03722A
Mills N (2006) ChemDraw Ultra 10.0 CambridgeSoft. 100 CambridgePark Drive, Cambridge, MA 02140. https://www.cambridgesoft.com
Davydov R, Strushkevich N, Smil D, Yantsevich A, Gilep A, Usanov S, Hoffman BM (2015) Evidence That Compound I Is the Active Species in Both the Hydroxylase and Lyase Steps by Which P450scc Converts Cholesterol to Pregnenolone: EPR/ENDOR/Cryoreduction/Annealing Studies. Biochem 54:7089–7097. https://doi.org/10.1021/acs.biochem.5b00903
Huang QQ, Yu WQ, Luo XL, Gao J, Xu J, Asian J (2018) Org Chem 7:2039–2044. https://doi.org/10.1002/ajoc.201800505
Hanson SK, Baker RT, Gordon JC, Scott BL, Thorn DL (2010) Aerobic Oxidation of Lignin Models Using a Base Metal Vanadium Catalyst. Inorg Chem 49:5611–5618. https://doi.org/10.1021/ic100528n
Hanson SK, Baker RT, Gordon JC, Scott BL, Sutton A, Thorn D (2008) J Am Chem Soc 131:428–429. https://doi.org/10.1021/ja807522n
Albert J, Lüders D, Bösmann A, Guldi DM, Wasserscheid P (2014) Spectroscopic and electrochemical characterization of heteropoly acids for their optimized application in selective biomass oxidation to formic acid. Green Chem 16:226–237. https://doi.org/10.1039/C3GC41320A
Wölfel R, Taccardi N, Bösmann A, Wasserscheid P (2011) Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen. Green Chem 13:2759. https://doi.org/10.1039/C1GC15434F
Criegee R, Kraft L, Rank B (1933) Die Glykolspaltung, ihr Mechanismus und ihre Anwendung auf chemische Probleme. Justus Liebigs Ann Chem 507:159–197
Heidt LJ, Gladding EK, Purves CB (1945) Paper Trade J 121:81
Buist GJ, Bunton CA (1954) The mechanism of oxidation of α-glycols by periodic acid. Part I. Ethylene glycol. Chem Soc 1406–1413. https://doi.org/10.1039/JR9540001406
Buist GJ, Bunton CA, Miles JH (1957) 919. The mechanism of oxidation of α-glycols by periodic acid. Part III. Spectroscopic evidence for the formation of an intermediate. Chem Soc 4575–4579. https://doi.org/10.1039/JR9570004575
Vennat M, Herson P, Brégeault JM, Shul’Pin M (2003) Vanadium-Catalysed Eur. J Inorg Chem 5:908–917
Khenkin AM, Neumann R (2008) Oxidative C−C Bond Cleavage of Primary Alcohols and Vicinal Diols Catalyzed by H 5 PV 2 Mo 10 O 40 by an Electron Transfer and Oxygen Transfer Reaction Mechanism. J Am Chem Soc 130:14474–14476. https://doi.org/10.1021/ja8063233
Brégeault JM (2003) Transition-metal complexes for liquid-phase catalytic oxidation: some aspects of industrial reactions and of emerging technologies. Dalton Trans 17:3289–3302. https://doi.org/10.1039/B303073N
Shaik S, Danovich D, Fiedler A, Schröder D, Schwarz H (1995) Two-State Reactivity in Organometallic Gas-Phase Ion Chemistry Helv. Chim Acta 78:1393–1407. https://doi.org/10.1002/hlca.19950780602
Schröder D, Shaik S, Schwarz H (2000) Two-State Reactivity as a New Concept in Organometallic Chemistry. Acc Chem Res 33:139–145. https://doi.org/10.1021/ar990028j
Shaik S, Hirao H (2007) Reactivity of High-Valent Iron–Oxo Species in Enzymes and Synthetic Reagents: A Tale of Many States. Acc Chem Res 40(7):532–542. https://doi.org/10.1021/ar600042c
de Visser SP, Ogliaro F, Harris N, Shaik S (2001) Multi-State Epoxidation of Ethene by Cytochrome P450: A Quantum Chemical Study. J Am Chem Soc 123:3037–3047. https://doi.org/10.1021/ja003544+
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revis. C.01, A.03, Gaussian, Inc., Wallingford CT
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615. https://doi.org/10.1039/B810189B
Chai, J.D., Head-Gordon, M.: Chem. Phys. 128, 084106 (2008). https://doi.org/10.1063/1.2834918
Hay PJ, Wadt WR (1985) Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J Chem Phys 82:270–283
Hamann DR (1989) Generalized norm-conserving pseudopotentials. Phys Rev B 40:2980–2987. https://doi.org/10.1103/PhysRevB.40.2980
Dolg M, Cao X (2011) Chem Rev 112:403–480. https://doi.org/10.1021/cr2001383
Ditchfield R, Hehre W, Pople J (1971) Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J Chem Phys 54:724–728
Check CE, Faust TO, Bailey JM, Wright BJ, Gilbert TM, Sunderlin LS (2001) Addition of Polarization and Diffuse Functions to the LANL2DZ Basis Set for P-Block Elements. J Phys Chem A 105:8111–8116. https://doi.org/10.1021/jp011945l
Tomasi, J., Menucci, B., Cammi, R.: ChemInform. 36, (2005). https://doi.org/10.1021/cr9904009
Maeda S, Harabuchi Y, Ono Y, Taketsugu T, Morokuma K (2014) Int J Quantum Chem 115:258–269. https://doi.org/10.1002/qua.24757
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. University Press, Cambridge
Jeffrey G (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Honda D, Ikegami S, Inoue T, Ozeki T, Yagasaki A (2007) Protonation and Methylation of an Anderson-Type Polyoxoanion [IMo6 O 24] 5. Inorg Chem 46:1464–1470. https://doi.org/10.1021/ic061881z
Alder K, Pascher F, Schmitz A (1943) Über die Anlagerung von Maleinsäure‐anhydrid und Azodicarbonsäure‐ester an einfach ungesättigte Koh an einfach ungesättigte Kohlenwasserstoffe. Zur Kenntnis von Substitutionsvorgängen in der Allyl‐Stellung. Ber Dtsch Chem Ges 76:27. https://doi.org/10.1002/cber.19430760105
Sarma B, Efremenko I, Neumann R (2015) Oxygenation of Methylarenes to Benzaldehyde Derivatives by a Polyoxometalate Mediated Electron Transfer–Oxygen Transfer Reaction in Aqueous Sulfuric Acid. J Am Chem Soc 137:5916–5922. https://doi.org/10.1021/jacs.5b01745
Efremenko I, Neumann R (2012) Computational Insight into the Initial Steps of the Mars–van Krevelen Mechanism: Electron Transfer and Surface Defects in the Reduction of Polyoxometalates. J Am Chem Soc 134:20669–20680. https://doi.org/10.1021/ja308625q
Lu T, Chen F (2011) J Comp Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graph 14(1):33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Author information
Authors and Affiliations
Contributions
M. A., M. Z., A. S., and M. S. formulated the scientific idea and planned the computations in this study. M. A. and M. Z. performed the computational calculations and prepared the first draft for this manuscript. A. S. and M. S. supervised the project. A. S. and M. S. edited and reviewed the manuscript prior to submission. All authors contributed to the article and approved the contents of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almi, M., Zhou, M., Saal, A. et al. Mechanistic insights into aerobic oxidative cleavage of glycol catalyzed by an Anderson-type polyoxometalate [IMo6O24]5−. J Mol Model 29, 57 (2023). https://doi.org/10.1007/s00894-023-05458-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05458-y