Abstract
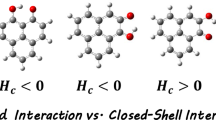
The eleven monohydrates of 1, 2, 3, 4-tetrahydroisoquinoline (THIQ) are analyzed through natural bond orbital (NBO) analysis and QTAIM methods employing M06-2X functional in DFT and MP2 methods. Here, the role of OH bonds as an acceptor and donor is critically analyzed. The role of lone pairs of O is critically monitored in two of the complexes, where N–H···O hydrogen bonds are present. The relative contributions of rehybridisation and hyperconjugation are compared in detail. Popelier criteria are satisfied in all the complexes barring a few exceptions involving weak hydrogen bonds. At the bond critical points (BCP), four monohydrates show higher values of electron density (ρC) and negative values of total electron energy density (HC), while Laplacian \({(\nabla }^{2}{\uprho }_{\mathrm{C}} )\) remains positive. These complexes satisfy the criteria of partial covalency. All these are O–H⋅⋅⋅N-type bonds. Remaining h-bonds are weaker in nature. These are also confirmed by the smaller values of ρC at the respective BCP. The variation of potential energy density (VC) among the complexes seems to be the most important factor in determining the nature of non-covalent interactions.









Similar content being viewed by others
References
Lewis GN (1916) The atom and the molecule. J Am Chem Soc 38:762–785
Kollman PA (1977) Noncovalent interactions. Chem Rev 10:365–371. https://doi.org/10.1021/ar50118a003
Lopinski GP, Wayner DDM, Wolkow RA (2000) Self‐directed growth of molecular nanostructures on silicon. Nature 406:48–51. https://doi.org/10.1038/35017519
Autumn K, Sitti M, Liang YA, Peattie AM, Hansen WR, Sponberg S, Kenny TW, Fearing R, Israelachvili JN, Full RJ (2002) Evidence for van der Waals adhesion in Gecko State. Proc Natl Acad Sci USA 99:12252
Li D, Zhu Z, Sun DW (2020) Visualization of the in situ distribution of contents and hydrogen bonding states of cellular level water in apple tissues by confocal Raman microscopy. Analyst 145:897–907. https://doi.org/10.1039/C9AN01743G
Etim EE, Gorai P, Das A, Chakrabarti SK, Arunan E (2008) Interstellar hydrogen bonding. Adv Space Res 612870–2880. https://doi.org/10.1016/j.asr.2018.03.003
Pauling L, Corey RB, Branson HR (1952) The structure of proteins: two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci USA 37:205
Watson JD, Crick FHC (1953) A structure for deoxyribose nucleic acid. Nature 171:737
Grabowski SJ (2011) What is the covalency of hydrogen bonding? Chem Rev 111:2597–2625. https://doi.org/10.1021/cr800346f
Mahadevi AS, Sastry GN (2016) Cooperativity in noncovalent interactions. Chem Rev 116:2775–2825. https://doi.org/10.1021/cr500344e
Zwier TS (1996) The spectroscopy of solvation in hydrogen-bonded aromatic clusters. Annu Rev Phys Chem 47:205–241. https://doi.org/10.1146/annurev.physchem.47.1.205
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P (2011) Definition of the hydrogen bond (IUPAC recommendations 2011. Pure Appl Chem 83:1637–1641
Ghosh S, Wategaonkar S (2020) C-H···Y (Y=N, O, π) Hydrogen bond: a unique unconventional hydrogen bond. J Ind Inst Sc 100:101–125. https://doi.org/10.1007/s41745-019-00145-5
Banerjee P, Chakraborty T (2018) Weak hydrogen bonds: insights from vibrational spectroscopic studies. Int Rev Phys Chem 37(1):83–123. https://doi.org/10.1080/0144235X.2018.1419731
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev Phys Chem 35(3):399–440. https://doi.org/10.1080/0144235X.2016.1192262
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) Electronic basis of improper hydrogen bonding: a subtle balance of hyperconjugation and rehybridization. J Am Chem Soc 125(19):5973–5987. https://doi.org/10.1021/ja034656e
Joseph J, Jemmis ED (2007) Red-, blue-, or no-shift in hydrogen bonds: a unifiedexplanation. J Am Chem Soc 129(15):4620–4632. https://doi.org/10.1021/ja067545z
Grabowski SJ (2013) Non-covalent interactions - QTAIM and NBO analysis. J Mol Model 19(11):4713–4721. https://doi.org/10.1007/s00894-012-1463-7
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893. https://doi.org/10.1021/cr00005a013
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford
(2007) Quantum theory of atoms in molecules: recent progress in theory and application; Matta, C., Boyd, R. J., Eds.; Wiley-VCH: New York.
Grabowski SJ, Sokalski WA, Leszczynski J (2006) The possible covalent nature of N-H….O hydrogen bonds in formamide dimer and related systems: an ab initio study. J Phys Chem A 110:4772–4779. https://doi.org/10.1021/jp055613i
Kuznetsov ML (2019) Relationships between interaction energy and electron density properties for homo halogen bonds of the [(A)nY–XX–Z(B)m] type (X = Cl, Br, I), Molecules 24:2733 https://doi.org/10.3390/molecules24152733
Koch U, Popelier PL (1995) Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747. https://doi.org/10.1021/j100024a016
Das S, Das L, Chakraborty A (2020) Conformers of 1,2,3,4 –tetrahydroisoquinoline in S0 and S1: an analysis through potential energy surface, hardness principles and vibrational spectroscopy. J Mol Struc 1207:127836. https://doi.org/10.1016/j.molstruc.2020.127836
Das S, Chakraborty A (2021) Conformer selective monohydrated clusters of 1,2,3,4 –tetrahydroisoquinoline in S0: I-Potential energy surface studies, vibrational signatures and NBO analysis. J Mol Struct 1225:129177. https://doi.org/10.1016/j.molstruc.2020.129177
Das S, Chakraborty A (2023) Computational investigation of the conformer selective complexes of 1,2,3,4-tetrahydroisoquinoline: ammonia (THIQ: NH3) in S0. J Mol Struct 134475. https://doi.org/10.1016/j.molstruc.2022.134475
Guchhait N, Banerjee S, Chakraborty A, Nath DN, Patwari GN, Chowdhury M (2004) Structure of hydrated clusters of tetrahydroisoquinoline THIQ–(H2O) n = 1,3 investigated by jet spectroscopy. J Chem Phys 120:9514–9523. https://doi.org/10.1063/1.1711810
Chakraborty A, Guchhait N, Banerjee S, Nath DN, Patwari GN, Chowdhury M (2001) Spectroscopic investigation of tetrahydroisoquinoline in supersonic jet. J Chem Phys 115:5184–5191. https://doi.org/10.1063/1.1394742
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Frisch MJ, Head-Gordon M, Pople JA (1990) A direct MP2 gradient method. Chem Phys Lett 166:275–280
Walker M, Harvey AJA, Sen A, Dessent CEH (2013) Performance of M06, M06–2X and M06-HF density functionals for conformationally flexible anionic clusters M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J Phys Chem A 117:12590–12600
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Gaussian09 (Revision B.1), Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakat-suji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T Jr, Montgomery JA, Peralta J E, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Bent HA (1961) An appraisal of valence-bond structures and hybridization in compounds of the first-row elements. Chem Rev 61:275–311
Johnson ER, Keinan S, Sa’nchez PM, Contreras-Garcı’a J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Fuster F, Silvi B (2000) Does the topological approach characterize the hydrogen bond? Theor Chem Acc 104:13–21
Emamian S, Lu T, Kruse H, Emamian H (2019) Exploring nature and predicting strength of hydrogen bonds: a correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J Comput Chem 9999:1–14. https://doi.org/10.1002/jcc.26068
Cubero E, Orozco M, Hobza P, Luque FJ (1999) Hydrogen bond versus anti-hydrogen bond: a comparative analysis based on the electron density topology. J Phys Chem A 103:6394. https://doi.org/10.1021/jp990258f
Parthasarathi R, Subramanian V, Sathyamurthy N (2005) Hydrogen bonding in phenol, water, and phenol-water clusters. J Phys Chem A 109:843–850. https://doi.org/10.1021/jp046499r
Kuznetsov ML (2019) Relationships between interaction energy and electron density properties for homo halogen bonds of the [(A)nY–XX–Z(B)m] type (X = Cl, Br, I), Molecules 24:2733. https://doi.org/10.3390/molecules24152733
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128(10):1527–1536. https://doi.org/10.1007/s12039-016-1172-3
Acknowledgements
AC gratefully acknowledges the support received from University Grants Commission through a research Project (F. No. 37-560/2009(SR)) for conducting this work. The authors also acknowledge the instrumental support from DST (Govt. of India) under the departmental FIST programme of the University of Burdwan (Grant no: SR/FST/PS-II-/2018/52(C)) and University Grants Commission (UGC) for departmental CAS (Grant no. F.530/20/CAS-II/2018(SAP-I)) scheme.
Author information
Authors and Affiliations
Contributions
Santu Das: literature survey, editing, software handling, data handling, presentation of figures. Abhijit Chakraborty: conceptualisation and visualisation of the problem, writing, reviewing and editing the manuscript, literature survey.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Chakraborty, A. Non-covalent interactions in the monohydrated complexes of 1,2,3,4–tetrahydroisoquinoline. J Mol Model 29, 37 (2023). https://doi.org/10.1007/s00894-022-05438-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05438-8