Abstract
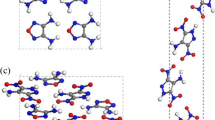
To effectively modify the strong hygroscopicity of ammonium dinitramide (ADN) crystal, the modification of ADN crystal in the 298 K under vacuum environment was studied through theoretical calculation. Three kinds of energetic nitramine molecules (X = RDX, HMX, and CL-20) were inserted into ADN crystal in different proportions (the molecular ratios of ADN to X are 6/1, 12/1, 18/1, and 24/1), to form a total of 12 kinds of designed ADN crystals. The results show that with the modification of ADN crystal with RDX, HMX, and CL-20, the crystal space group, cell parameters, crystal density, and growth morphology will be changed under vacuum conditions. According to the analyses of adsorption heat data, four proportional modification systems all reduced the hygroscopicity of ADN crystal to varying degrees. It is worth noting that the hygroscopicity of modified ADN crystal tends to decrease with the increase of the proportion of doping molecules, but the stability gradually deteriorates, especially 18ADN/1CL-20 and 24ADN/1CL-20. Although they have an excellent anti-moisture effect, from the perspective of crystal energy stability, the actual syntheses of these two kinds of crystal cells are the most difficult. Combined with the energy stability and hygroscopicity analysis, 1HMX/24ADN crystal is a more suitable anti-hygroscopicity modification scheme among the doped ADN crystals. In this case, the isothermal adsorption heat of ADN crystal decreases from 0.692 kcal/mol to 0.573 kcal/mol. The theoretical simulation study of ADN doping modification in a vacuum will provide significant references for ADN modification in the actual situation.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Wang S, Xiao L, Hu Y, Zhang G, Gao H, Zhao F, Hao G, Jiang W (2021) A review on the preparation and application of nano-energetic materials. Chin J Explos Propellants 44(6):705–734. https://doi.org/10.14077/j.issn.1007-7812.202112013
Lan Y, Zhai J, Li D, Yang R (2015) The influence of solution chemistry on the morphology of ammonium dinitramide crystals. J Mater Sci 50(14):4933–4939. https://doi.org/10.1007/s10853-015-9040-y
Wingborg N (2006) Ammonium dinitramide-water: interaction and properties. J Chem Eng Data 51(5):1582–1586
Cui J, Han J, Wang J, Huang R (2010) Study on the Crystal Structure ADN Hygroscopicity of Ammonium Dinitramide. J Chem Eng Data 55(9):3229–3234. https://doi.org/10.1021/je100067n
Chen F, Xuan C, Lu Q, Xiao L, Yang J, Hu Y, Zhang G, Wang Y, Zhao F, Hao G, Jiang W (2022) A review on the high energy oxidizer ammonium dinitramide: Its synthesis, thermal decomposition, hygroscopicity, and application in energetic materials. Def Technol. https://doi.org/10.1016/j.dt.2022.04.006
Li J, Yang R, Zeng T, Jinghui Hu, Tang W, Liu Z, Gong Li (2021) Preparation and growth mechanism of micro spherical ammonium dinitramide crystal based on ultrasound-assisted solvent-antisolvent method. Ultrason Sonochem 78:105716. https://doi.org/10.1016/J.ULTSONCH.2021.105716
Bellas MK, Matzger AJ (2019) Achieving balanced energetics through cocrystallization. Angew Chem 131(48):17345–17348. https://doi.org/10.1002/ange.201908709
Rahman A, Chin J, Cheah KH (2008) Prilling and coating of ammonium dinitramide (ADN) solid green propellant in toluene mixture using ultrasound sonication. Aerospace 5(1):29. https://doi.org/10.3390/aerospace5010029
Chen X, He L, Li X, Zhou Z, Ren Z (2019) Molecular simulation studies on the growth process and properties of ammonium dinitramide crystal. J Phys Chem C 123(17):10940–10948. https://doi.org/10.1021/acs.jpcc.9b00120
Qiangqiang L, Chen F, Xiao L, Yang J, Yubing H, Zhang G, Zhao F, Wang Y, Jiang W, Hao G (2022) Advances in the molecular simulation and numerical calculations of the green high-energy oxidant ADN. Mater Today Commun 31:103699. https://doi.org/10.1016/j.mtcomm.2022.103699
Ren Z, Chen X, Guojia Yu, Wang Y, Chen B, Zhou Z (2020) Molecular simulation studies on the design of energetic ammonium dinitramide co-crystals for tuning hygroscopicity. CrystEngComm 22(31):5237–5244. https://doi.org/10.1039/d0ce00602e
Xu Y, Zhou Y, Wang X, Zhang W, Ma E, Deringer VL, Mazzarello R (2022) Unraveling crystallization mechanisms and electronic structure of phase-change materials by large-scale ab initio simulations. Adv Mater 34(11):e2109139. https://doi.org/10.1002/ADMA.202270084
Morgan EE, Evans HA, Pilar K, Brown CM, Clément RJ, Maezono R, Seshadri R, Monserrat B, Cheetham AK (2022) Lattice Dynamics in the NASICON NaZr2(PO4)3 solid electrolyte from temperature-dependent neutron diffraction, nmr, and ab initio computational studies. Chem Mater 34(9):4029–4038. https://doi.org/10.1021/ACS.CHEMMATER.2C00212
Hao J, Xinlu C (2019) MD simulation of methane adsorption properties on pillared graphene bubble models. J Mol Model 25(8):236. https://doi.org/10.1007/s00894-019-4132-2
Howard RH, Meunier M (2019) Molecular modelling with materials studio®. CRC Press, Boca Raton
Thanikaivelan P, Padmanabhan J, Subramanian V, Ramasami T (2002) Chemical reactivity and selectivity using Fukui functions: basis set and population scheme dependence in the framework of B3LYP theory. Theoret Chem Acc 107(6):326–335. https://doi.org/10.1007/s00214-002-0352-z
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113(18):7756. https://doi.org/10.1063/1.1316015
Delley B (2010) Time dependent density functional theory with DMol3. J Phys: Condens Matter 22(38):384208–384214. https://doi.org/10.1088/0953-8984/22/38/384208
Becke Axel D (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. https://doi.org/10.1063/1.464913
Lee TJ, Handy NC, Rice JE, Schaefer HF (1986) The efficient evaluation of configuration interaction analytic energy second derivatives: Application to hydrogen thioperoxide, HSOH. J Chem Phys 85(7):3930–3938. https://doi.org/10.1063/1.451826
Zhao Y, Geng WT, Freeman AJ, Delley B (2002) Structural, electronic, and magnetic properties of α- and β-MnAs: LDA and GGA investigations. Phys Rev B 65(11):113202–113203. https://doi.org/10.1103/PhysRevB.65.113202
Basiuk VA, Henao-Holguín LV (2014) Dispersion-corrected density functional theory calculations of meso -tetraphenylporphine-C60 complex by using DMol3 module. J Comput Theor Nanosci 11(7):1609–1615. https://doi.org/10.1166/jctn.2014.3539
Beltrán A, Gracia L, Andrés J (2006) Density functional theory study of the brookite surfaces and phase transitions between natural titania polymorphs. J Phys Chem B 110(46):23417–23423. https://doi.org/10.1021/jp0643000
Taylor CR, Bygrave PJ, Hart JN, Allan NL, Manby FR (2012) Improving density functional theory for crystal polymorph energetics. Phys Chem Chem Phys 14(21):7739–7743. https://doi.org/10.1039/c2cp24090d
Akkermans RLC, Spenley NA, Robertson SH (2013) Monte carlo methods in materials studio. Mol Simul 39(14–15):1153–1164. https://doi.org/10.1080/08927022.2013.843775
Zhou T, Chen F, Li J, He L, Ren Y, Wang X, Cao D, Wang J (2020) Morphology prediction of 5,5′-bistetrazole-1,1′-diolate (BTO) crystal in solvents with different models using molecular dynamics simulation. J Cryst Growth 548:125843. https://doi.org/10.1016/j.jcrysgro.2020.125843
Hartman P, Bennema P (1980) The attachment energy as a habit controlling factor: I. Theoretical considerations. J Cryst Growth 49(1):145–156. https://doi.org/10.1016/0022-0248(80)90075-5
Himeno S, Takenaka M, Shimura S (2008) Light gas adsorption of all-silica DDR- and MFI-type zeolite: computational and experimental investigation. Mol Simul 34(10–15):1329–1336. https://doi.org/10.1080/08927020802411703
Vincenzo C, Susanna M (2006) Peptide-TiO2 surface interaction in solution by ab initio and molecular dynamics simulations. J Phys Chem B 110(12):6160–6169. https://doi.org/10.1021/jp056760j
Gabriel M, Alberto V, Thomas H (2005) Description of electron delocalization via the analysis of molecular fields. Chem Rev 105(10):3812–3841. https://doi.org/10.1021/cr030086p
Sandhya KS, Suresh CH (2014) Designing metal hydride complexes for water splitting reactions: a molecular electrostatic potential approach. Dalton Trans 43(32):12279–12287. https://doi.org/10.1039/c4dt01343c
Pingale SS (2011) Molecular electrostatic potential for exploring π-conjugation: a density-functional investigation. Phys Chem Chem Phys 13(33):15158–15165. https://doi.org/10.1039/C1CP20071B
Bayoumy AM, Ibrahim M, Omar A (2020) Mapping molecular electrostatic potential (MESP) for fulleropyrrolidine and its derivatives. Opt Quant Electron 52(7):77–86. https://doi.org/10.1007/s11082-020-02467-6
Bolotina NB, Hardie MJ, Speer RL Jr, Pinkerton AA (2004) Energetic materials: variable-temperature crystal structures of γ-and∊-HNIW polymorphs. J Appl Crystallogr 37(5):808–814. https://doi.org/10.1107/S0021889804017832
Zhu W, Xiao J, Zhu W, Xiao H (2009) Molecular dynamics simulations of RDX and RDX-based plastic-bonded explosives. J Hazard Mater 164(2–3):1082–1088. https://doi.org/10.1016/j.jhazmat.2008.09.021
Cobbledick RE, Small RWH (1975) The crystal structure of the complex formed between 1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetraazacyclooctane (HMX) and N, N-dimethylformamide (DMF). Acta Crystallogr Sect B: Struct Crystallogr Cryst Chem 31(12):2805–2808. https://doi.org/10.1107/S056774087500893X
Gilardi R, Anderson JF, George C, Butcher RJ (1997) A new class of flexible energetic salts: the crystal structures of the ammonium, lithium, potassium, and cesium salts of dinitramide. J Am Chem Soc 119(40):9411–9416. https://doi.org/10.1021/ja9709280
Price SL (2008) From crystal structure prediction to polymorph prediction: interpreting the crystal energy landscape. Phys Chem Chem Phys 10(15):1996–2009. https://doi.org/10.1039/b719351c
Martín-Islán AP, Martín-Ramos D, Sainz-Díaz CI (2008) Crystal structure of minoxidil at low temperature and polymorph prediction. J Pharm Sci 97(2):815–830. https://doi.org/10.1002/jps.21015
Um J, McFarquhar GM, Hong YP, Lee S-S, Jung CH, Lawson RP, Mo Q (2015) Dimensions and aspect ratios of natural ice crystals. Atmos Chem Phys 15(7):3933–3956. https://doi.org/10.5194/acp-15-3933-2015
Bellucci L, Cavallucci T, Tozzini V (2019) From the buffer layer to graphene on silicon carbide: Exploring morphologies by computer modeling. Front Mater. https://doi.org/10.3389/fmats.2019.00198
Macedonia MD, Maginn EJ (1999) A biased grand canonical Monte Carlo method for simulating adsorption using all-atom and branched united atom models. Mol Phys 96(9):1375–1390. https://doi.org/10.1080/00268979909483082
Veniamin Z, Aleksey G, Tatiana S, Sergey I (2021) Modeling of sorption kinetics of U(VI) micro-quantities nanostructured materials with anatase mesoporous structures. Radiochim Acta 109(9):653–660. https://doi.org/10.1515/RACT-2021-1031
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Project Nos. 21805139, 21905023, 12102194, 22005144, and 22005145), the Joint Funds of the National Natural Science Foundation of China (U2141202), Natural Science Foundation of Jiangsu Province (BK20200471), the Fundamental Research Funds for the Central Universities (No. 30920041106, 30921011203), and Young Elite Scientists Sponsorship Program by CAST (Program, 2021QNRC001).
Funding
This work was financially supported by the National Natural Science Foundation of China (Project Nos. 21805139, 21905023, 12102194, 22005144 and 22005145), the Joint Funds of the National Natural Science Foundation of China (U2141202), Natural Science Foundation of Jiangsu Province (BK20200471), the Fundamental Research Funds for the Central Universities (No. 30920041106, 30921011203), and Young Elite Scientists Sponsorship Program by CAST (Program, 2021QNRC001).
Author information
Authors and Affiliations
Contributions
Qiangqiang Lu contributed to investigation; data curation; and writing—original draft. Lei Xiao and Yinglei Wang performed validation and conceptualization. Guangpu Zhang and Yubing Hu carried out formal analysis. Fuyao Chen and Fengqi Zhao were involved in design of methodology and creation of models. Junqing Yang performed writing—review and editing. Wei Jiang contributed to acquisition of the financial support for the project leading to this paper. Gazi Hao took management and coordination responsibility for the research activity planning and execution; and formulation of overarching research goals and aims.
Corresponding authors
Ethics declarations
Conflicts of interest/Competing interests
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, Q., Xiao, L., Wang, Y. et al. Theoretical simulation study on crystal property and hygroscopicity of ADN doping with nitramine explosives (RDX, HMX, and CL-20). J Mol Model 28, 208 (2022). https://doi.org/10.1007/s00894-022-05200-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05200-0