Abstract
Much research has been done on reactions of a single CO2 molecule with a graphene surface. In this paper, density functional theory calculations are used to investigate the adsorption and reaction of double CO2 on the surface of single vacancy (SV) and divacancy (DV) defect graphene. The study found that due to the mutual repulsion between CO2 and the size of the SV defect, it is difficult for two CO2 molecular to be adsorbed directly above the SV defect at the same time. Regardless of SV or DV, the adsorption of the first CO2 in the defect center will have a beneficial effect on the adsorption of the second CO2. In addition, the transition state calculation of the CO2 reaction on the DV plane was carried out, and the adsorption behavior was analyzed and studied. This in-depth study is helpful to the understanding of the reaction behavior of CO2 on graphene, and further exploration in the direction of the effective application of graphene to the reaction and adsorption of CO2.
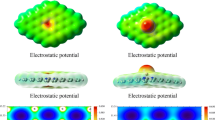
Graphical abstract
Our work explores the adsorption behavior of CO2 on graphene surfaces, the physical and chemical adsorption of double CO2 at the defect was studied and analyzed.










Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sun C, Bai B (2017) Molecular sieving through a graphene nanopore: non-equilibrium molecular dynamics simulation. Sci Bull 08(v.62):34–42
Hu H, Yu J, Li Y et al (2011) Engineering of a novel pluronic F127/graphene nanohybrid for pH responsive drug delivery. J Biomed Mater Res A 100A(1):141–148
Lin Y, Tian Y, Sun H et al (2020) Progress in modifications of 3D graphene-based adsorbents for environmental applications. Chemosphere 270:129420
Kudahi SN, Noorpoor AR, Mahmoodi NM (2017) Determination and analysis of CO2 capture kinetics and mechanisms on the novel graphene-based adsorbents. J CO2 Util 21:17–29
Meng LY, Park SJ (2012) Effect of exfoliation temperature on carbon dioxide capture of graphene nanoplates. J Colloid Interface Sci 386(1):285–290
Sevanthi R, Irin F, Parviz D et al (2016) Electrical current stimulated desorption of carbon dioxide adsorbed on graphene based structures. RSC Adv 6(49):43401–43407
Balasubramanian R, Chowdhury S (2015) Recent advances and progress in the development of graphene-based adsorbents for CO2 capture. J Mater Chem A 3(44):21968–21989
Murugan L, Lakshmipathi S, Bhatia SK (2014) Influence of in-plane Stone–Thrower–Wales defects and edge functionalisation on the adsorption of CO2 and H2O on graphene. RSC Adv 4(74):39576–39587
Leslie-Fernanda, Velázquez-López, Sandy-María et al (2019) DFT study of CO adsorption on nitrogen/boron doped-graphene for sensor applications. J Mol Model
Murugan L, Lakshmipathi S (2017) Gas adsorption efficacy of graphene sheets functionalised with carboxyl, hydroxyl and epoxy groups in conjunction with Stone–Thrower–Wales (STW) and inverse Stone–Thrower–Wales (ISTW) defects. Phys Chem Chem Phys. https://doi.org/10.1039/C1037CP06900F
Jin CH, Lan HP, Peng LM et al (2009) Deriving carbon atomic chains from graphene. Phys Rev Lett 102(20):205501
Banhart F, Kotakoski J, Krasheninnikov AV (2011) Structural defects in graphene. ACS Nano 5(1):26–41
Paul RK, Badhulika S, Saucedo NM et al (2012) Graphene nanomesh as highly sensitive chemiresistor gas sensor. Anal Chem 84(19):8171–8178
Cabrera-Sanfelix P (2009) Adsorption and reactivity of CO2 on defective graphene sheets. J Phys Chem A
Liu Y, Wilcox J (2011) CO2 Adsorption on carbon models of organic constituents of gas shale and coal. Environ Sci Technol 45(2):809
Montoya A, Mondragón F, Truong TN (2003) CO2 adsorption on carbonaceous surfaces: a combined experimental and theoretical study. Carbon 41(1):29–39
(2018) Enhancement of CO2 adsorption on oxygen-functionalized epitaxial graphene surface under near-ambient conditions. Phys Chem Chem Phys 20(29): 19532–19538
Lalitha M, Lakshmipathi S, Bhatia SK (2017) Edge functionalised & Li-intercalated 555–777 defective bilayer graphene for the adsorption of CO2 and H2O. Appl Surf Sci 400(1):375–390
Wu H-Z, Bandaru S, Liu J et al (2018) Adsorption of H2O, H2, O2, CO, NO, and CO2 on graphene/g-C3N4 nanocomposite investigated by density functional theory. Appl Surf Sci 430(1):125–136
Hwang DG, Jeong E, Lee SG (2016) Density functional theory study of CH4 and CO2 adsorption by fluorinated graphene. Carbon Lett 20(1):81–85
Del C, Calles AG, Raúl E et al (2018) Adsorption of CO2 on graphene surface modified with defects. Comput Condens Matter 16:e00315
Giannozzi P, Baroni S, Bonini N et al (2009) Quantum ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21(39):395502
Scandolo S, Giannozzi P, Cavazzoni C et al (2005) First-principles codes for computational crystallography in the Quantum-ESPRESSO package. Z Kristallogr 220(5):574–579
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188
Akilan R, Malarkodi M, Vijayakumar S et al (2019) Modeling of 2-D hydrogen-edge capped defected & boron-doped defected graphene sheets for the adsorption of CO2, SO2 towards energy harvesting applications. Appl Surf Sci 463(JAN.1):596–609
Robertson AW, Lee G-D, He K et al (2014) Stability and dynamics of the tetravacancy in graphene. Nano Lett 14(3):1634–1642
Zheng P, Zhang X, Duan Y et al (2020) Oxidation of graphene with variable defects: alternately symmetrical escape and self-restructuring of carbon rings. Nanoscale 12(18):10140–10148
Hashimoto A, Suenaga K, Gloter A et al (2004) Direct evidence for atomic defects in graphene layers. Nature 430(7002):870–873
Bao Z-Q, Shi J-J, Yang M et al (2011) Magnetism induced by D3-symmetry tetra-vacancy defects in graphene. Chem Phys Lett 510(4–6):246–251
Yamashita K, Saito M, Oda T (2006) Atomic geometry and stability of mono-, di-, and trivacancies in graphene. Jpn J Appl Phys 45(8R):6534
Chen N, Yang RT (1998) Ab initio molecular orbital study of the unified mechanism and pathways for gas− carbon reactions. J Phys Chem A 102(31):6348–6356
Chen N, Yang RT (1998) Ab initio molecular orbital calculation on graphite: selection of molecular system and model chemistry. Carbon 36(7–8):1061–1070
Montoya A, Mondragon F, Truong TN (2002) First-principles kinetics of CO desorption from oxygen species on carbonaceous surface. J Phys Chem A 106(16):4236–4239
Montoya A, Truong T-TT, Mondragon F et al (2001) CO desorption from oxygen species on carbonaceous surface: 1. Effects of the local structure of the active site and the surface coverage. J Phys Chem A 105(27):6757–6764
Chen S, Yang R, Kapteijn F et al (1993) A new surface oxygen complex on carbon: toward a unified mechanism for carbon gasification reactions. Ind Eng Chem Res 32(11):2835–2840
Tit N, Said K, Mahmoud NM et al (2017) Ab-initio investigation of adsorption of CO and CO2 molecules on graphene: role of intrinsic defects on gas sensing. Appl Surf Sci 394(feb.1):219–230
Montoya A, Mondragón F, Truong TN (2002) Formation of CO precursors during char gasification with O2, CO2 and H2O. Fuel Process Technol 77(25):125–130
Maziar N (2016) DFT study on the sensitivity of open edge graphene toward CO2 gas. Vacuum 194–200
Ghosh A, Subrahmanyam KS, Krishna KS et al (2008) Uptake of H2 and CO2 by graphene. J Phys Chem C 112(40):15704–15707
Li K, Khanna R, Zhang H et al (2021) Thermal behaviour, kinetics and mechanisms of CO2 interactions with graphene: an atomic scale reactive molecular dynamic study. Chem Eng J 425(131529):1385–8947
Liang Z, Li K, Wang Z et al (2021) Adsorption and reaction mechanisms of single and double H2O molecules on graphene surfaces with defects: a density functional theory study. Phys Chem Chem Phys
Zandiatashbar A, Lee GH, An SJ et al (2014) Effect of defects on the intrinsic strength and stiffness of graphene. Nat Communications 53186
Funding
The authors acknowledge the financial support of the financial support from the Young Elite Scientist Sponsorship Program by CAST (YESS20210090), and the National Natural Science Foundation of China (51974019, 51804025 and 51774032). All calculations were performed with the support of the Niagara supercomputer at the SciNet HPC Consortium in the Compute/Calcul Canada national computing platform. SciNet is funded by the Canada Foundation for Innovation under the auspices of Compute Canada, the Government of Ontario, Ontario Research Fund—Research Excellence, and the University of Toronto. The authors acknowledge Prof. Mansoor Barati of the University of Toronto for the technical support.
Author information
Authors and Affiliations
Contributions
Shujie Zhang: computation, simulation, analysis, and draft writing. Kejiang Li: analysis and interpretation of data for the work and modify the manuscript. Zeng Liang: discussion, modify the manuscript, and participate in discussions. Jianliang Zhang: investigation, supervision, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
894_2022_5105_MOESM1_ESM.docx
Supplementary file1. The adsorption energies of four kinds of physical adsorption structures on the plane: original graphene surface; SW surface defects; Single vacancy defect surface; Double vacancy defect surface, the data in bold in the table is the optimal adsorption energy. (DOCX 35 KB)
Rights and permissions
About this article
Cite this article
Zhang, S., Liang, Z., Li, K. et al. A density functional theory study on the adsorption reaction mechanism of double CO2 on the surface of graphene defects. J Mol Model 28, 118 (2022). https://doi.org/10.1007/s00894-022-05105-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05105-y