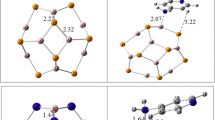
Abstract
Using density functional theory, the adsorption of valproic acid onto the surface of fullerene-like nanocages was investigated. Valproic acid interacts with the nanocages through the carboxylic group with energies of − 144.14, − 109.71, − 105.22, and − 84.96 kcal/mol. The frontier molecular orbital (FMO) energy levels were considerably altered upon adsorption, resulting in a reduction in energy gap and increase in electrical conductivity. This suggests that nanocages could be used as sensors as well as options for drug administration in biological systems. Solvation effects in water are also reported.
Graphical abstract








Similar content being viewed by others
Data availability
The supplementary information files include additional materials.
Code availability
No new codes have been created. Existing codes were utilized and quoted correctly.
References
Beheshtian J, Peyghan AA, Noei M (2013) Sensing behavior of Al and Si doped BC3 graphenes to formaldehyde. Sens Actuators, B Chem 181:829–834. https://doi.org/10.1016/j.snb.2013.02.086
Rad AS, Samipour V, Movaghgharnnezhad S, Mirabi A, Shahavi MH, Moghadas BK (2019) X12N12 (X=Al, B) cluster for protection of vitamin C: molecular modeling investigation. Surface Interfaces 15:30–37. https://doi.org/10.1016/j.surfin.2019.02.001
Almuqrin AH, Al-Otaibi JS, Mary YS, Mary YS, Thomas R (2021) Structural study of letrozole and metronidazole and formation of self assembly with graphene and fullerene with the enhancement of physical, chemical and biological activities. J Biomol Struct Dyn 39:5509–5515. https://doi.org/10.1080/07391102.2020.1790420
Al-Otaibi JS, Mary YS, Thomas R, Kaya S (2020) Detailed electronic structure, physico-chemical properties, excited state properties, virtual bioactivity screening and SERS analysis of three guanine based antiviral drugs, valacyclovir HCl hydrate, Acyclovir and ganciclovir. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2020.1773876
Al-Otaibi JS, Mary YS, Mary YS, Kaya S, Erkan S (2020) Spectral analysis and DFT investigation of some benzopyran analogues and their self assemblies with graphene. J Mol Liq 317:113924. https://doi.org/10.1016/j.molliq.2020.113924
Rad AS, Ayub K (2017) Adsorption properties of acetylene and ethylene molecules onto pristine and nickel decoarated Al12N12 nanoclsuters. Mater Chem Phys 194:337–344. https://doi.org/10.1016/j.matchemphys.2017.04.002
Rad AS, Ayub K (2017) DFT study of boron trichloride adsorption on the surface of Al12N12 nanocluster. Mol Phys 115:879–884. https://doi.org/10.1080/00268976.2017.1290843
Velammal SP, Devi TA, Amaladhas TP (2016) Antioxidant, antimicrobial and cytotoxic activities of silver and gold nanoparticle ssynthesized using plumbago zylanica bark. J Nanostruct Chem 6:247–260. https://doi.org/10.1007/s40097-016-0198-x
Goodarzi Z, Maghrebi M, Zavareh AF, Mokhtari-Hosseini ZB, Ebrahimi-Hoseinzadeh B, Zarmi AH, Barshan-Tashnizi M (2015) Evaluation of nicotine sensor based on copper nanoparticles and carbon nanotubes. J Nanostruct Chem 5:237–242. https://doi.org/10.1007/s40097-015-0154-1
Harismah K, Mirzaei M, Samadizadeh M, Rad AS (2017) DFT studies of stabilities and properties for X3Y6Z9 borazine like structures (X=B/Al, Y-N/P, Z=H/Me). Superlattices Microstruct 109:360–365. https://doi.org/10.1016/j.spmi.2017.05.027
Jafari Z, Baharfar R, Rad AS, Asghari S (2020) Potential of graphene oxide as a drug delivery system for sumatriptan: a detailed density functional theory study. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1736161
Khodashenas B, Ardjmand M, Mazyar SB, Rad AS, Khiyavi AA (2019) Bovine serum albumin/gold nanoparticles as a drug delivery system for curcumin: experimental an dcomputational studies. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2019.1683073
Padash R, Rahimi-Nasrabadi M, Rad AS, Sobhani-Nasab A, Jesionowski T, Ehrlich H (2019) A comparative computational investigation of phosgene adsorption on (XY)12 (X=Al, B and Y=N, P) nanoclusters: DFT investigations. J Cluster Sci 30:203–218. https://doi.org/10.1007/s10876-018-1479-y
Padash R, Sobhani-Nasab A, Rahimi-Nasrabadi M, Mirmotahri M, Enrlich H, RAd AS, Peyravi M (2018) Is it possible to use X12Y12 (X=Al, B and Y=N, P) nanocages for drug delivery systems? A DFT study on the adsorption property of 4-aminopyriidne drug. Appl Phys A 124:582. https://doi.org/10.1007/s00339-018-1965-y
Sherfati M, Rad AS, Ardjmand M, Heydarinasab A, Peyravi M, Mirzaei M (2018) Beryllium oxide (BeO) nanotube provides excellent surface towards adenine adsorption: a dispersion-corrected DFT study in gas and water phases. Curr Appl Phys 18:1059–1065. https://doi.org/10.1016/j.cap.2018.05.024
Strout DL (2000) Structure and stability of boron nitrides: isomers of B12N12. J Phys Chem A 104:3364–3366. https://doi.org/10.1021/jp994129a
Wang R, Zhang D, Liu C (2005) Theoretical prediction of a novel inorganic fullerene like family of silicon carbon materials. Chem Phys Lett 411:333–338. https://doi.org/10.1016/j.cplett.2005.06.055
Loscher W (2002) Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 16:669–694. https://doi.org/10.2165/00023210-200216100-00003
Waszkielewicz AM, Gunia A, Sloczynska K, Marona H (2011) Evaluation of anticonvulsants for possible use in neuropathic pain. Curr Med Chem 18:4344–4358. https://doi.org/10.2174/092986711797200408
Chiu CT, Wang Z, Hunsberger JG, Chuang DM (2013) Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105–142. https://doi.org/10.1124/pr.111.005512
Hoffmann J, Akerman S, Goadsby PJ (2014) Efficacy and mechanism of anticonvulsant drugs in migraine. Expert Rev Clin Pharmacol 7:191–201. https://doi.org/10.1586/17512433.2014.885835
Ornoy A (2009) Valproic acid in pregnancy: how much are we endangering the embryo and fetus? Reprod Toxicol 28:1–10. https://doi.org/10.1016/j.reprotox.2009.02.014
Alver O, Parlak C, Senyel M, Ramasami P (2018) Density functional theory study on the adsorption of valproic acid to doped fullerenes. Main Group Met Chem 41:67–71. https://doi.org/10.1515/mgmc-2018-0002
Da Ros T (2008) Twenty years of promises: Fullerene in medicinal chemistry. In: F Cata; dp. T Da Ros (eds), Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes, Carbon Materials: Chemistry and Physics, vol.1, Springer, Dordrecht, https://doi.org/10.1007/978-1-4020-6845-4_1
Singh R, Lillard JW (2009) Nanoparticle based targeted drug delivery. Exp Mol Pathol 86:215–223. https://doi.org/10.1016/j.yexmp.2008.12.004
Zakharian TY, Seryshev A, Sithraman B, Gilbert BE, Knight V, Wilson LJ (2005) A fullerene paclitaxel chemotherapeutic: synthesis, characterization and study of biological activity in tissue culture. J Am Chem Soc 127:12508–12509. https://doi.org/10.1021/ja0546525
Ashcroft JM, Tsyboulski DA, Hartman KB, Zakharian TY, Marks JW, Weisman RB, Rosenblum MG, Wilson LJ (2006) Fullerene (C60) immunoconjugates: interaction of water soluble C60 derivatives with the murine anti-gp240 melanoma antibody. Chem Commun (Camb) 28:3004–3006. https://doi.org/10.1039/B601717G
Gaussian 16, Revision A.03, M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A.V. Marenich, J. Bloino, B.G. Janesko, R. Gomperts, B. Mennucci, H.P. Hratchian, J.V. Ortiz, A.F. Izmaylov, J.L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V.G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J.A. Montgomery, Jr., J.E. Peralta, F. Ogliaro, M.J. Bearpark, J.J. Heyd, E.N. Brothers, K.N. Kudin, V.N. Staroverov, T.A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A.P. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, J.M. Millam, M. Klene, C. Adamo, R. Cammi, J.W. Ochterski, R.L. Martin, K. Morokuma, O. Farkas, J.B. Foresman, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016.
GaussView, Version 6.1, R.Dennington, T.A.Keith, J.M.Millam, Semichem Inc., Shawnee Mission, KS, 2016.
Mahboobeh K, Elham TL (2020) B12Y12 (Y:N, P) fullerene like cages for emestane-delivery; molecular modeling investigation. J Mol Struct 1217:128455. https://doi.org/10.1016/j.molstruc.2020.128455
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Fuentealba P, Simon-Manso Y, Chattaraj PK (2000) Molecular electronic excitations and the minimum polarizability principle. J Phys Chem A 104:3185–3187. https://doi.org/10.1021/jp992973v
Padash R, Esfahani MR, Rad AS. The computational quantum mechanical study of sulfamide drug adsorption ontoX12Y12 fullerene like nanocages: detailed DFT and QTAIM investigations. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1292991
Mary YS, Kumar VS, Mary YS, Resmi KS, Thomas R (2020) Detailed quantum mechanical studies on three bioactive benzimidazole derivatives and their Raman enhancement on adsorption over graphene sheets. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2020.1852267
Al-Otaibi JS, Almuqrin AH, Mary YS, Mary YS (2020) Utilization of O/S-doped graphene nanoclusters for ultrasensitive detection of flurane derivatives-DFT investigations. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1870155
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comp Chem 24:669–681. https://doi.org/10.1002/jcc.10189
Almuqrin AH, Al-Otaibi JS, Mary YS, Thomas R, Kaya S, Isin DO (2020) Spectral analysis and detailed quantum mechanical investigation of some acetanilide analogues and their self assemblies with graphene and fullerene. J Mol Model 26:254. https://doi.org/10.1007/s00894-020-04485-3
Al-Otaibi JS, Almuqrin AH, Mary YS, Mary YS, Van Alsenoy C (2020) DFT and molecular docking studies of self-assembly of sulfone analogues and graphene. J Mol Model 26:273. https://doi.org/10.1007/s00894-020-04546-7
O’boyle NM, Tenderholt AL, Langner KM (2008) CClib: a library for package independent computational chemistry algorithms. J Comput Chem 29:839–845. https://doi.org/10.1002/jcc.20823
Al-Otaibi JS, Almuqrin AH, Mary YS, Mary YS, Thomas R (2020) Modeling the conformational preference, spectral analysis and other quantum mechanical studies on three bioactive aminobenzoate derivatives and their SERS active graphene complexes. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2020.1827270
Mary YS, Mary YS, Armakovic S, Armakovic SJ, Narayana B (2020) Understanding reactivity of a triazole derivative and its interaction with graphene and doped/undoped coronene- a DFT study. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1837677
Almuqrin AH, Al-Otaibi JS, Mary YS, Mary YS (2021) DFT computational study towards investigating psychotropic drugs, promazine and trifluoperazine adsorption on graphene, fullerene and carbon cyclic ring nano clusters. Spectrochim Acta 246:119012. https://doi.org/10.1017/j.saa.2020.119012
Al-Otaibi JS, Mary YS, Mary YS, Serdaroglu G (2021) Adsorption of adipic acid in Al/B-N/P nanocages: DFT investigations. J Mol Model 27:113. https://doi.org/10.1007/s00894-021-04742-z
Al-Otaibi JS, Mary YS, Mary YS, Kaya S, Serdaroglu G (2020) DFT computational study of trihalogenated aniline derivative’s adsorption onto graphene/fullerene/fullerene-like nanocages, X12Y12 (X=Al, B and Y=N, P). J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2021.1914172
Rad AS (2015) First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl Surf Sci 357:1217–1224. https://doi.org/10.1016/j.apsusc.2015.09.168
Zou M, Zhang J, Chen J, Li X (2012) Simulating adsorption of organic pollutants on finite (8,0) single walled carbon nanotubes in water. Environ Sci Technol 46:8887–8894. https://doi.org/10.1021/es301370f
Al-Otaibi JS, Mary YS, Mary YS (2022) Adsorption of a thione bioactive derivative over different silver/gold clusters – DFT investigations. Comput Theor Chem 1207:113497. https://doi.org/10.1016/j.comptc.2021.113497
Al-Otaibi JS, Mary YS, Mary YS, Ullah Z, Yadav R, Gupta N, Churchill DG (2021) Adsorption properties of dacarbazine with graphene/fullerene/metal nanocages-reactivity, spectroscopic and SERS analysis. Spectrochim Acta 120677. https://doi.org/10.1016/j.saa.2021.120677
Mary YS, Mary YS, Ullah Z (2021) Computational study of sorbic acid drug adsorption onto coronene/fullerene/fullerene-like X12Y12 (X=Al, B and Y=N, P) nanocages: DFT and molecular docking investigations. J Cluster Sci. https://doi.org/10.1007/s10876-021-02106-4
Al-Otaibi JS, Mary YS, Mary YS, Thomas R (2022) Evidence of cluster formation of croconic acid with Ag, Au and Cu cages, enhancement of electronic properties and Raman activity. Spectrochim Acta 264:120233. https://doi.org/10.1016/j.saa.2021.120233
Al-Otaibi JS, Mary YS, Mary YS, Trivedi R, Chakraborty B (2021) Theoretical investigation on the adsorption of melamine in Al12/B12-N12/P12 fullerene-like nanocages: a platform for ultrasensitive detection of melamine. Chem Pap. https://doi.org/10.1007/s11696-021-01849-8
Al-Otaibi JS, Mary YS, Mary YS, Ullah Z, Kwon HW (2022) Adsorption behavior and solvent effects of an adamantane-triazole derivative on metal clusters-DFT simulation studies. J Mol Liq 345:118242. https://doi.org/10.1016/j.molliq.2021.118242
Acknowledgements
The authors express their gratitude to Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R13), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Jamelah S. Al-Otaibi: software, supervision, manuscript preparation and data analysis. Y. Sheena Mary: supervision, manuscript preparation, conceiving the problem and data analysis. Y. Shyma Mary: manuscript preparation, conceiving the problem, and data analysis and correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Otaibi, J.S., Mary, Y.S. & Mary, Y.S. DFT analysis of valproic acid adsorption onto Al12/B12-N12/P12 nanocages with solvent effects. J Mol Model 28, 98 (2022). https://doi.org/10.1007/s00894-022-05088-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05088-w