Abstract
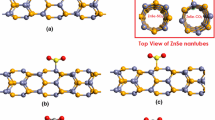
In this work, the interaction of GaN nanotube (GaNNT) with common air pollutants of industrialized cities, such as NH3, NO2 and SO2 in different configurations was studied. For this study, the single-walled (10,0) GaNNT was used. The analysis was done via the density functional theory implemented in the SIESTA simulation software. The analysis of the results shows that the air pollutants alter the properties of nanotubes when they interact with them. The stability analysis shows that the most stable configurations are those in which adsorption occurs through a chemical process. The systems remain semiconductors, but in the case of NO2 and SO2 molecules interacting with GaNNT, there was a significant reduction in the energy gap. Our results also indicate that GaNNT is a promising material to detect and remove NH3 and NO2 molecules from the environment; however, it may be not applicable to detect or remove SO2, because the latter interacts strongly with the nanotube, which prevents the GaNNT from being reused.









Similar content being viewed by others
References
Khan MS, Srivastava A (2016) . J Electroanal Chem 775:243
Jiang R, Guo W, Li M, Zhu H, Li J, Zhao L, Fu D, Sham H (2009) . J Phys Chem C 113:18223
Wu Z, Hahm MG, Jung YJ, Menon L (2009) . J Mater Chem 19:463
Xie Y, Huo Y-P, Zhang J-M (2012) . Appli Surf Sci 258:6391
Zhao J, Buldum A, Han J, Lu P (2002) . Nanotechnology 13:195
Baei MT, Soltani AR, Moradi AV, Lemeski ET (2011) . Comput Theor Chem 970:30
Wu RQ, Yang M, Lu YH, Feng YP, Huang ZG, Wu QY (2008) . J Phys Chem C 112:15985
An W, Wu X, Zeng XC (2008) . J Phys Chem C 112:57647
Lin F, Zhou G, Li Z, Wu J, Duan W (2009) . Chem Phys Lett 475:82
Ganji MD (2008) . Phys Lett A 372:3277
Kang SM, Shin TI, Dinh DV, Yang JH, Kim SW, Yoon Dh (2009) . Microelectron J 40:373
Lee S, Lee YH, Hwang YG, Elsner J, Porezag D, Frauenheim T (1999) . Phys Rev B 60:S253
Lee S, Lee YH, Hwang YG, Lee CJ (1999) . J Korean Phys Soc 34(3):S253–S257
Goldberger J, He R, Zhang Y, Lee S, Yan H, Choi H-J, Yang P (2003) . Nature 422:599
Colussi ML, Baierle RJ, Miwa RH (2008) . J Appl Phys 104:033712
Khan MS, Srivastava A (2016) . J Electroanaly Chem 775:243
Frisch MJ, et al. (2009) Gaussian09 revision a, vol 02. Gaussian, Inc., Wallingford
Hohenberg P, Kohn W (1964) . Phy Rev 136:864B
Ordejon P, Soler JM (1996) . Phys Rev B 53:10441
Kohn W, Sham LJ (1965) . Phys Rev 140:A1133
Troullier N, Martins JL (1991) . Phys Rev B 43:1993
Kleinman L, Bylander DM (1982) . Phys Rev Lett 48:1425
Artacho E, Sachez-Portal D, Ordejon P, Garcia A, Soler JM (1999) . Phys Status Solidi B 215:809
Monkhorstand HJ, Pack JD (1976) . Phys Rev B 13:5188
Perdew JP, Burke K, Ernzerhof M (1996) . Phys Rev B 77:5048
Boys SF, Bernardi F (1970) . Mol Phys 19:553
Sodré JM, Longo E, Talf CA, Martins JBB, Santos JD (2017) . Compt Rend Chim 20:190
Ribeiro CC, Varela Junior J, Guerini S (2018) . J Mol Model 24:192
Machado FM, Carmalin SA, Lima EC, Dias SLP, Prola LDT, Saucier C, Jauris IM, Zanella I, Fagan SB (2016) . J Phys Chem C 120:18296
Machado FM, Bergmann CP, Lima EC, Royer B, Souza FE, Jauris IM, Calveted T, Fagan SB (2012) . Phys Chem Chem Phys 14:11139
Mülliken RS (1955) . J Chem Phys 23:1833
Acknowledgements
The authors acknowledge CENAPAD-SP and LSIM-UFMA for computer times. Andrew A. J. Anchieta da Silva acknowledge to CAPES for the fellowship.
Funding
The authors acknowledge FAPEMA and CAPES for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Author contribution
A. J. Anchieta da Silva performed all the calculations presented in this study. All analyzes were performed by Silvete Guerini and A. J. Anchieta da Silva. The first draft of the manuscript was written by Silvete Guerini and Caio Vinícius Caetano. All authors contributed to the study conception and interpretation of results. The writing and revision of the final version of the manuscript were performed by Caio Vinícius Caetano.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, A.A.J.A., Caetano, C.V. & Guerini, S. Energetic and electronic properties of NH3, NO2 and SO2 interacting with GaN nanotube: a DFT study. J Mol Model 27, 234 (2021). https://doi.org/10.1007/s00894-021-04826-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04826-w