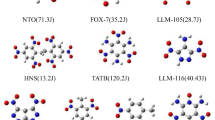
Abstract
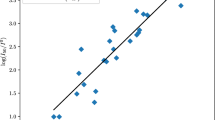
The quantification of bond strengths is a useful and general concept in chemistry. In this work, a Coulombic force model based on atomic electric charges computed using the accurate distributed multipole analysis (DMA) partition of the molecular charge density was employed to quantify the weakest N–NO2 and C–NO2 bond strengths of 19 nitrobenzene, 11 nitroazole, and 10 nitramine molecules. These bonds are known as trigger linkages because they are usually related to the initiation of an explosive. The three families of explosives combine different types of molecular properties and structures ranging from essentially aromatic molecules (nitrobenzenes) to others with moderate aromaticity (nitroazoles) and non-aromatic molecules with cyclic and acyclic skeletons (nitramines). We used the results to investigate the impact sensitivity of the corresponding explosives employing the trigger linkage concept. For this purpose, the computed Coulombic bond strength of the trigger linkages was used to build four sensitivity models that lead to an overall good agreement between the predicted values and available experimental sensitivity values even when the model included the three chemical families simultaneously. We discussed the role of the trigger linkages for determining the sensitivity of the explosives and rationalized eventual discrepancies in the models by examining alternative decomposition mechanisms and features of the molecular structures.
Graphical abstract

















Similar content being viewed by others
Data availability
All the data can be found in the Supporting Information.
References
McWeeny R, Coulson CA (1980) Coulson's Valence. Oxford University Press, Oxford
Cremer D, Wu AN, Larsson A, Kraka E (2000) Some thoughts about bond energies, bond lengths, and force constants. J Mol Model 6(4):396–412
de Sousa DWO, Nascimento MAC (2017) Are one-electron bonds any different from standard two-electron covalent bonds? Acc Chem Res 50(9):2264–2272
Fantuzzi F, Cardozo TM, Nascimento MAC (2017) On the metastability of doubly charged homonuclear diatomics. Phys Chem Chem Phys 19(29):19352–19359
Bader RFW (1994) Atoms in molecules: a quantum theory. International series of monographs on chemistry, vol 22. Clarendon Press, Oxford
Politzer P, Murray JS (2019) A look at bonds and bonding. Struct Chem 30(4):1153–1157
Romanova A, Lyssenko K, Ananyev I (2018) Estimations of energy of noncovalent bonding from integrals over interatomic zero-flux surfaces: correlation trends and beyond. J Comput Chem 39(21):1607–1616
Su XF, Cheng XL, Meng CM, Yuan XL (2009) Quantum chemical study on nitroimidazole, polynitroimidazole and their methyl derivatives. J Hazard Mater 161(1):551–558
Turker L, Atalar T (2006) Quantum chemical study on 5-nitro-2,4-dihydro-3H-1,2,4-triazol-3-one (NTO) and some of its constitutional isomers. J Hazard Mater 137(3):1333–1344
Jun Z, Xin-lu C, Bi H, Xiang-dong Y (2006) Neural networks study on the correlation between impact sensitivity and molecular structures for nitramine explosives. Struct Chem 17(5):501–507
Song XS, Cheng XL, Yang XD, Li DH, Linghu RF (2008) Correlation between the bond dissociation energies and impact sensitivities in nitramine and polynitro benzoate molecules with polynitro alkyl groupings. J Hazard Mater 150(2):317–321
Atalar T, Jungova M, Zeman S (2009) A new view of relationships of the N-N bond dissociation energies of cyclic nitramines. Part II. Relationships with Impact Sensitivity. J Energ Mater 27(3):200–216
Kraka E, Cremer D (2009) Characterization of CF bonds with multiple-bond character: bond lengths, stretching force constants, and bond dissociation energies. ChemPhysChem 10(4):686–698
Sacks LJ (1986) Coulombic models in chemical bonding: 1. Description and some applications of a Coulombic model. J Chem Educ 63(4):288–296
Sacks LJ (1986) Coulombic models in chemical bonding: 2. Dipole-moments of binay hydrides. J Chem Educ 63(5):373–376
Fried LE, Manaa MR, Pagoria PF, Simpson RL (2001) Design and synthesis of energetic materials. Annu Rev Mater Res 31:291–321
Kuklja MM, Stefanovich EV, Kunz AB (2000) An excitonic mechanism of detonation initiation in explosives. J Chem Phys 112(7):3417–3423
Dippold AA, Klapotke TM (2013) A study of Dinitro-bis-1,2,4-triazole-1,1 '-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N-oxides. J Am Chem Soc 135(26):9931–9938
Klapotke TM, Krumm B, Widera A (2018) Synthesis and properties of Tetranitro-substituted adamantane derivatives. ChemPlusChem 83(1):61–69
Badgujar DM, Talawar MB, Asthana SN, Mahulikar PP (2008) Advances in science and technology of modern energetic materials: an overview. J Hazard Mater 151(2–3):289–305
Trache D, Tarchoun AF (2018) Stabilizers for nitrate ester-based energetic materials and their mechanism of action: a state-of-the-art review. J Mater Sci 53(1):100–123
Sivabalan R, Anniyappan M, Pawar SJ, Talawar MB, Gore GM, Venugopalan S, Gandhe BR (2006) Synthesis, characterization and thermolysis studies on triazole and tetrazole based high nitrogen content high energy materials. J Hazard Mater 137(2):672–680
Nanayakkara S, Kraka E (2019) A new way of studying chemical reactions: a hand-in-hand URVA and QTAIM approach. Phys Chem Chem Phys 21(27):15007–15018
Politzer PM, J. S. (2003) Energetic materials. Part 1. Decomposition, crystal and molecular properties, vol 12. Theoretical and Computational Chemistry. Elsevier, Amsterdan
Politzer PM, J. S. (2003) Energetic materials. Part 2. Detonation, Combustion., vol 13. Theoretical and Computational Chemistry. Elsevier, Amsterdan
Shaw RWB, T. B., Thompson DL (2005) Overviews of recent research on energetic materials, vol 16. Advanced Series in Physical Chemistry, World Scientific, New Jersey
Zeman S, Jungova M (2016) Sensitivity and performance of energetic materials. Propellants Explos Pyrotech 41(3):426–451
Politzer P, Murray JS (2016) High performance, low sensitivity: conflicting or compatible? Propellants Explos Pyrotech 41(3):414–425
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106(9):1770–1783
Zeman S (2007) Sensitivities of high energy compounds. High energy density materials, vol 125. Structure and Bonding. Springer-Verlag, Berlin, Berlin, pp 195–271
Mathieu D (2013) Toward a physically based quantitative modeling of impact sensitivities. J Phys Chem A 117(10):2253–2259
Mathieu D, Alaime T (2014) Predicting impact sensitivities of nitro compounds on the basis of a semi-empirical rate constant. J Phys Chem A 118(41):9720–9726
Mathieu D (2015) Prediction of gurney parameters based on an analytic description of the expanding products. J Energ Mater 33(2):102–115
Rae PJ, Dickson PM (2020) Some observations about the drop-weight explosive sensitivity test. J Dyn Behav Mater
Kamlet MJ The relationship of impact sensitivity with structure of organic high explosives. 1. Polynitroaliphatic explosives. In: 6th International Symposium on Detonation, San Diego, California, 1976. ONR Report ACR 221, p 312
Kamlet MJ, Adolph HG (1979) Relationship of impact sensitivity with structure of organic high explosives: . polynitroaromatic explosives. Propellants and Explosives 4(2):30–34
Mathieu D (2016) Physics-based modeling of chemical hazards in a regulatory framework: comparison with quantitative structure-property relationship (QSPR) methods for impact sensitivities. Ind Eng Chem Res 55(27):7569–7577
Zhang CY (2009) Review of the establishment of nitro group charge method and its applications. J Hazard Mater 161(1):21–28
Murray JS, Concha MC, Politzer P (2009) Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C-NO2/N-NO2 bond dissociation energies. Mol Phys 107(1):89–97
Yan QL, Zeman S (2013) Theoretical evaluation of sensitivity and thermal stability for high explosives based on quantum chemistry methods: a brief review. Int J Quantum Chem 113(8):1049–1061
Li G, Zhang C (2020) Review of the molecular and crystal correlations on sensitivities of energetic materials. J Hazard Mater 398:122910
Owens FJ, Jayasuriya K, Abrahmsen L, Politzer P (1985) Computational analysis of some properties associated with the nitro groups in polynitroaromatic molecules. Chem Phys Lett 116(5):434–438
Politzer P, Murray JS (1996) Relationships between dissociation energies and electrostatic potentials of C-NO2 bonds: applications to impact sensitivities. J Mol Struct 376:419–424
Murray JS, Lane P, Politzer P (1998) Effects of strongly electron-attracting components on molecular surface electrostatic potentials: application to predicting impact sensitivities of energetic molecules. Mol Phys 93(2):187–194
Ren FD, Cao DL, Shi WJ, Gao HF (2016) A theoretical prediction of the relationships between the impact sensitivity and electrostatic potential in strained cyclic explosive and application to H-bonded complex of nitrocyclohydrocarbon. J Mol Model 22(4):8
Politzer P, Murray JS, Serninario JM, Lane P, Grice ME, Concha MC (2001) Computational characterization of energetic materials. Theochem-J Mol Struct 573:1–10
Rice BM, Sahu S, Owens FJ (2002) Density functional calculations of bond dissociation energies for NO2 scission in some nitroaromatic molecules. Theochem-J Mol Struct 583:69–72
Li JS (2010) Relationships for the impact sensitivities of energetic C-nitro compounds based on bond dissociation energy. J Phys Chem B 114(6):2198–2202
Li J (2010) A quantitative relationship for the shock sensitivities of energetic compounds based on X–NO2 (X=C, N, O) bond dissociation energy. J Hazard Mater 180(1):768–772
Song XS, Cheng XL, Yang XD, He B (2006) Relationship between the bond dissociation energies and impact sensitivities of some nitro-explosives. Propellants Explos Pyrotech 31(4):306–310
Mathieu D (2012) Theoretical shock sensitivity index for explosives. J Phys Chem A 116(7):1794–1800
Mathieu D (2017) Sensitivity of energetic materials: theoretical relationships to detonation performance and molecular structure. Ind Eng Chem Res 56(29):8191–8201
Keshavarz MH (2010) Simple relationship for predicting impact sensitivity of nitroaromatics, nitramines, and nitroaliphatics. Propellants Explos Pyrotech 35(2):175–181
Keshavarz MH (2013) A new general correlation for predicting impact sensitivity of energetic compounds. Propellants Explos Pyrotech 38(6):754–760
Keshavarz MH, Ghaffarzadeh M, Omidkhah MR, Farhadi K (2017) New correlation between electric spark and impact sensitivities of nitramine energetic compounds for assessment of their safety. Z Anorg Allg Chem 643(19):1227–1231
Keshavarz MH, Ghaffarzadeh M, Omidkhah MR, Farhadi K (2017) Correlation between shock sensitivity of nitramine energetic compounds based on small-scale gap test and their electric spark sensitivity. Z Anorg Allg Chem 643(24):2158–2162
Keshavarz MH, Abadi YH (2018) Novel organic compounds containing nitramine groups suitable as high-energy cyclic nitramine compounds. ChemistrySelect 3(28):8238–8244
Shoaf AL, Bayse CA (2018) Trigger bond analysis of nitroaromatic energetic materials using wiberg bond indices. J Comput Chem 39(19):1236–1248
Stone AJ (1981) Distributed multipole analysis, or how to describe a molecular charge-distribution. Chem Phys Lett 83(2):233–239
Stone AJ, Alderton M (1985) Distributed multipole analysis - methods and applications. Mol Phys 56(5):1047–1064
Stone AJ (2000) The theory of intermolecular forces. International Series of Monographs on Chemistry. Oxford University Press, Oxford
Stone AJ (2005) Distributed multipole analysis: stability for large basis sets. J Chem Theory Comput 1(6):1128–1132
Borges I (2008) Conformations and charge distributions of diazocyclopropanes. Int J Quantum Chem 108(13):2615–2622
Anders G, Borges I (2011) Topological analysis of the molecular charge density and impact sensitivy models of energetic molecules. J Phys Chem A 115(32):9055–9068
Oliveira RSS, Borges Jr I (2020) Correlation between molecular charge properties and impact sensitivity of explosives: nitrobenzene derivatives. Propellants, Explosives, Pyrotechnics (at press)
Moraes TF, Borges I (2011) Nuclear Fukui functions and the deformed atoms in molecules representation of the electron density: application to gas-phase RDX (hexahydro-1,3,5-trinitro-1,3,5- triazine) electronic structure and decomposition. Int J Quantum Chem 111 (7–8):1444–1452
Giannerini T, Borges I (2015) Molecular electronic topology and fragmentation onset via charge partition methods and nuclear Fukui functions: 1,1-diamino-2,2-dinitroethylene. J Braz Chem Soc 26(5):851–859
Oliveira MAS, Borges I (2019) On the molecular origin of the sensitivity to impact of cyclic nitramines. Int J Quantum Chem 119(8):14
de Oliveira RSS, Borges I (2019) Correlation between molecular charge densities and sensitivity of nitrogen-rich heterocyclic nitroazole derivative explosives. J Mol Model 25(10):314
Borges I, Silva AM, Aguiar AP, Borges LEP, Santos JCA, Dias MHC (2007) Density functional theory molecular simulation of thiophene adsorption on MoS2 including microwave effects. Theochem-J Mol Struct 822(1–3):80–88
Borges I, Silva AM (2012) Probing topological electronic effects in catalysis: thiophene adsorption on NiMoS and CoMoS clusters. J Braz Chem Soc 23(10):1789–1799
Borges I, Silva AM, Modesto-Costa L (2018) Microwave effects on NiMoS and CoMoS single-sheet catalysts. J Mol Model 24(6):8
Silva AM, Borges I (2011) How to find an optimum cluster size through topological site properties: Mosx model clusters. J Comput Chem 32(10):2186–2194
Dlott DD (2003) Fast molecular processes in energetic materials. In: Politzer P, Murray JS (eds) energetic materials. Part 2. Detonation, combustion, vol 12. Elsevier, Amsterdam,
Brill TB, James KJ (1993) Thermal decomposition of energetic material. 61. Perfidy in the amini-2,4,6-trinitrobenzene series of explosives. J Phys Chem 97(34):8752–8758
Pro S (1999). Wavefunction, Irvine
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev B 136 (3B):B864-&
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):1133–1138
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98(2):1372–1377
Frisch MJ (et al., Gaussian 03, Revision B.01, 2003) Gaussian 03, Revision B.01
Price SL, Stone AJ (1983) A distributed multipole analysis of charge-densities of the azabenzene molecules. Chem Phys Lett 98(5):419–423
Atkins PW, dePaula J (2006) Physical chemistry8th edn. W. H, Freeman and Company, New York (NY)
Politzer P, Murray JS, Lane P, Sjoberg P, Adolph HG (1991) Shock-sensitivity relationships for nitramines and nitroaliphatics. Chem Phys Lett 181(1):78–82
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Wiley Interdiscip Rev-Comput Mol Sci 1(2):153–163
Clark T, Murray JS, Politzer P (2018) A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys Chem Chem Phys 20(48):30076–30082
Politzer P, Murray JS, Clark T, Resnati G (2017) The sigma-hole revisited. Phys Chem Chem Phys 19(48):32166–32178
Politzer P, Murray JS (2018) The Hellmann-Feynman theorem: a perspective. J Mol Model 24(9):7
Xiang D, Zhu WH (2017) Thermal decomposition of isolated and crystal 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowurtzitane according to ab initio molecular dynamics simulations. RSC Adv 7(14):8347–8356
Storm CB, Stine JR, Kramer JF (1990) In: chemistry and physics of energetic materials. Kluwer Acad. Publ, Dordrecht, pp 605–640
Fischer JW, Hollins RA, LoweMa CK, Nissan RA, Chapman RD (1996) Synthesis and characterization of 1,2,3,4-cyclobutanetetranitramine derivatives. J Organomet Chem 61(26):9340–9343
Yau AD, Byrd EFC, Rice BM (2009) An investigation of KS-DFT electron densities used in atoms-in-molecules studies of energetic molecules. J Phys Chem A 113(21):6166–6171
Dubovitskii FI, Korsunskii BL (1981) Kinetics of the thermal decomposition ofN-nitro-compounds. Russ Chem Rev 50(10):958–978
Astakhov AM, Dyugaev KP, Kuzubov AA, Nasluzov VA, Vasiliev AD, Buka ÉS (2009) Theoretical studies of the structure of nitrimines. I. Structure of 2-nitroguanidine and its alkyl derivatives. J Struct Chem 50(2):201–211
Bracuti AJ (1999) Crystal structure refinement of nitroguanidine. J Chem Crystallogr 29(6):671–676
Moxnes JF, Froyland O, Risdal T (2017) A computational study of ANTA and NTO derivatives. J Mol Model 23(8):8
Schmidt NB, Lee GS, Mitchell AR, Gilardi R (2001) Synthesis and properties, of a new explosive, 4-amino-3,5-d i n it ro-1 H-Pyrazole(LLM-116). Lawrence Livermore National Laboratory, Lawrence Livermore National Laboratory
Bulusu NB (1990) Chemistry and physics of energetic materials, vol 309. 1st edn. Kluwer Academic Publishers, Dordrecht
Depaz JLG, Ciller J (1994) Structure and tautomerismo of anta (aminonitrotriazole). Propellants Explos Pyrotech 19(1):32–41
Su XF, Cheng XL, Ge SH (2009) Theoretical investigation on structure and properties of 2,4,5-trinitroimidazole and its three derivatives. Theochem-J Mol Struct 895(1–3):44–51
Damavarapu R, Surapaneni CR, Duddu RG, Zhang M, Dave PR (2007) Melt-cast explosive material. United States Patent,
Kalescky R, Kraka E, Cremer D (2013) Local vibrational modes of the formic acid dimer – the strength of the double hydrogen bond. Mol Phys 111(9–11):1497–1510
Tao Y, Zou W, Kraka E (2017) Strengthening of hydrogen bonding with the push-pull effect. Chem Phys Lett 685:251–258
Freindorf M, Kraka E, Cremer D (2012) A comprehensive analysis of hydrogen bond interactions based on local vibrational modes. Int J Quantum Chem 112(19):3174–3187
Politzer P, Seminario JM, Bolduc PR (1989) A proposed interpretation of the destabilizing effect of hydroxyl-groups of nitroaromatic molecules. Chem Phys Lett 158(5):463–469
Murray JS, Lane P, Politzer P, Bolduc PR (1990) A relationship between impact sensitivy and the electrostatic potentials at the midpoints of C-NO2 bonds in nitroaromatics. Chem Phys Lett 168(2):135–139
Zhang CY, Shu YJ, Huang YG, Zhao XD, Dong HS (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109(18):8978–8982
Acknowledgments
We thank an anonymous referee of another publication [68] for suggesting to pursue further a Coulombic force for modeling the sensitivity of explosives. The support of the Brazilian Army to this work through our Institute is greatly acknowledged.
Funding
Support for this research is from the Brazilian agency CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through research grants 304148/2018-0 and 409447/2018-8. R. S. S. O. has a Ph.D. scholarship from Capes (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
The Gaussian, Sparta, and the GDMA2 (http://www-stone.ch.cam.ac.uk/pub/gdma/) programs were used to generate the data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
The online version of this article has additional Supplementary Material that includes acronyms of the molecules; monopole values and Coulombic force values of the N-NO2 and C-NO2 bonds; normalized Coulombic force values for the analyzed molecules and converged geometry data for the nitramine and nitroazole molecules. (DOCX 4167 kb)
Rights and permissions
About this article
Cite this article
Oliveira, M.A.S., Oliveira, R.S.S. & Borges, I. Quantifying bond strengths via a Coulombic force model: application to the impact sensitivity of nitrobenzene, nitrogen-rich nitroazole, and non-aromatic nitramine molecules. J Mol Model 27, 69 (2021). https://doi.org/10.1007/s00894-021-04669-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04669-5