Abstract
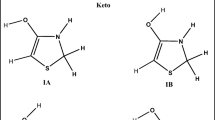
The single-electron transfer (SET) reactions from the neutral and mono-anion species of five imidazole alkaloids (lepidines A, B, C, D, and E) against hydroperoxyl radicals have been studied using the density functional theory and the Marcus theory. The deprotonated species of three alkaloids were found to have free radical scavenging activity. The antioxidant activity was studied via single-electron transfer (SET) under physiological conditions. The SET reactions for lepidines B, D, and E were found to have rate constants ranging from 105 to 106 M−1 s−1. Therefore, they are predicted to be able to deactivate hydroperoxyl radicals and therefore the damage caused by them to polyunsaturated fatty acids. It is important to mention that the acid-base equilibrium plays an important role in their free radical scavenging activity.

Lepidines are predicted to be able to deactivate hydroperoxyl radicals and the damage caused by them to polyunsaturated fatty acids.





Similar content being viewed by others
References
Hernandez J, Goycoolea FM, Quintero J, Acosta A, Castañeda M, Dominguez Z, Robles R, Vazquez-Moreno L, Velazquez EF, Astiazaran H, Lugo E, Velazquez C (2007) Sonoran propolis: chemical composition and antiproliferative activity on cancer cell lines. Planta Med 73:1469–1474
Matus MH, Domínguez Z, Salas-Reyes M, Hernández J, Cruz-Sánchez S (2010) Conformational study of caffeic acid derivatives. J Mol Struct: THEOCHEM 953:175–181
Aksu K, Topal F, Gulcin I, Tümer F, Göksu F (2015) Acetylcholinesterase inhibitory and antioxidant activities of novel symmetric sulfamides derived from phenethylamines. Arch Pharm Chem Life Sci 348:446–455
Gülçin I, Oktay M, Küfrevioğlu OI, Aslan A (2002) Determination of antioxidant activity of lichen Cetraria islandica (L). Ach J Ethnopharmacol 79:325–329
Shahidi F, Zhong Y (2010) Lipid oxidation and improving the oxidative stability. Chem Soc Rev 39:4067–4079
Zou L, Akoh CC (2015) Antioxidant activities of annatto and palm tocotrienol-rich fractions in fish oil and structured lipid-based infant formula emulsion. Food Chem 168:504–511
Halliwell B, Murcia MA, Chirico S, Aruoma OI (1995) Free radicals and antioxidants in food and in vivo: what they do and how they work. Crit Rev Food Sci Nutr 35:7–20
Halake K, Birajdar M, Lee J (2016) Structural implications of polyphenolic antioxidants. J Ind Eng Chem 35:1–7
Rice-Evans CA, Miller J, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Sepúlveda-Jiménez G, Porta-Ducoing H, Rocha-Sosa M (2003) La participación de los metabolitos secundarios en la defensa de las plantas. Rev Mex Fitopatol 21:355–363
Facchini PJ (2001) ALKALOID BIOSYNTHESIS IN PLANTS: Biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Physiol Plant Mol Biol 52:29–66
Erol NT, Sarı F, Velioglu YS (2010) Polyphenols, alkaloids and antioxidant activity of different grades Turkish black tea. GIDA 35:161–168
Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah H (2008) Antioxidant activity of some common plants. Turk J Biol 32:51–55
Chung HS, Shin JC (2007) Characterization of antioxidant alkaloids and phenolic acids from anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem 104:1670–1677
Costa EV, da Cruz PE, de Lourenco CC, de Souza Moraes VR, de Lima Nogueira PC, Salvador MJ (2013) Antioxidant and antimicrobial activities of aporphinoids and other alkaloids from the bark of Annona salzmannii A. DC. (Annonaceae). Nat Prod Res 27:1002–1006
Jung HA, Min BS, Yokozawa T, Lee JH, Kim YS, Choi JS (2009) Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol Pharm Bull 32:1433–1438
Cui B, Zheng BL, He K, Zheng QY (2003) Imidazole alkaloids from Lepidium meyenii. J Nat Prod 66:1101–1103
Sandoval M, Okuhama NN, Angeles FM, Melchor VV, Condezo LA, Lao J, Miller MJS (2002) Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii). Food Chem 79:207–213
Aydemir T, Becerik S (2011) Phenolic content and antioxidant activity of different extracts from Ocinum basilicum, Apium Graveolens and Lepidium sativum seeds. J Food Biochem 35:62–79
Cuentas R, De la Cruz L, Hernández G, Mateo I, Castañeda C, Ibáñez L, Ramos E (2008) Evaluación del efecto antioxidante de hojas de Lepidium peruvianum Chacón,“maca”. Rev Horizonte Médico 8:45–55
Zia-Ul-Haq M, Ahmad S, Calani L, Mazzeo T, Del Rio D, Pellegrini N, De Feo V (2012) Compositional study and antioxidant potential of Ipomoea hederacea Jacq. and Lepidium sativum L. seeds. Molecules 17:10306–10321
Yamamoto Y, Kuwahara T, Watanabe K, Watanabe K (1996) Antioxidant activity of 3-methyl-1-phenyl-2-pyrazolin-5-one. Redox Rep 2:333–338
Abe S, Kirima K, Tsuchiya K, Okamoto M, Hasegawa T, Houchi H, Yoshizumi M, Tamaki T (2004) The reaction rate of edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one (MCI-186)) with hydroxyl radical. Chem Pharm Bull 52:186–191
Nakagawa H, Ohyama R, Kimata A, Suzuki T, Miyata N (2006) Hydroxyl radical scavenging by edaravone derivatives: efficient scavenging by 3-methyl-1-(pyridin-2-yl)-5-pyrazolone with an intramolecular base. Bioorg Med Chem Lett 16:5939–5942
Pérez-Gonzalez A, Galano A (2011) OH radical scavenging activity of edaravone: mechanism and kinetics. J Phys Chem B 115:1306–1314
Galano A, Alvarez-Idaboy JR (2013) A computational methodology for accurate predictions of rate constants in solution: application to the assessment of primary antioxidant activity. J Comput Chem 34:2430–2445
Zhang D, Liu Y, Chu L, Wei Y, Wang D, Cai S, Zhou F, Ji B (2013) Relationship between the structures of flavonoids and oxygen radical absorbance capacity values: a quantum chemical analysis. J Phys Chem A 117:1784–1794
Markovic Z, Milenkovic D, Dorovic J, Dimitric-Markovic JM, Stepanic V, Lucic B, Amic D (2012) PM6 and DFT study of free radical scavenging activity of morin. Food Chem 134:1754–1760
Amic D, Lucic B (2010) Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorg Med Chem 18:28–35
Lespade L, Bercion S (2012) Theoretical investigation of the effect of sugar substitution on the antioxidant properties of flavonoids. Free Radic Res 46:346–358
Mohajeri A, Asemani SS (2009) Theoretical investigation on antioxidant activity of vitamins and phenolic acids for designing a novel antioxidant. J Mol Struct 930:15–20
Terpinc P, Abramovic H (2010) A kinetic approach for evaluation of the antioxidant activity of selected phenolic acids. Food Chem 121:366–371
Itagaki S, Kurokawa T, Nakata C, Saito Y, Oikawa S, Kobayashi M, Hirano T, Iseki K (2009) In vitro and in vivo antioxidant properties of ferulic acid: a comparative study with other natural oxidation inhibitors. Food Chem 114:466–471
De Grey ADNJ (2002) HO2•: the forgotten radical. DNA Cell Biol 21:251–257
Bielski BH, Arudi RL, Sutherland MW (1983) A study of the reactivity of HO2/O2-with unsaturated fatty acids. J Biol Chem 258:4759–4761
Frisch MJ et al (2009) Gaussian 09 revision B.01. Gaussian Inc., Wallingford
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Zavala-Oseguera C, Alvarez-Idaboy JR, Merino G, Galano A (2009) OH radical gas phase reactions with aliphatic ethers: a variational transition state theory study. J Phys Chem A 113:13913–13920
Galano A, Alvarez-Idaboy JR (2014) Kinetics of radical-molecule reactions in aqueous solution: a benchmark study of the performance of density functional methods. J Comput Chem 35:2019–2026
Zhao Y, Truhlar DG (2008) How well can new-generation density functionals describe the energetics of bond-dissociation reactions producing radicals? J Phys Chem A 112:1095–1099
Galano A, Pérez-González A, Castañeda-Arriaga R, Muñoz-Rugeles L, Mendoza-Sarmiento G, Romero-Silva A, Ibarra-Escutia A, Rebollar-Zepeda AM, Leon-Carmona JR, Hernández-Olivares MA, Alvarez-Idaboy JR (2016) Empirically fitted parameters for calculating pKa values with small deviations from experiments using a simple computational strategy. J Chem Inf Model 56:1714–1724
Pérez-González A, Galano A (2011) Ionization energies, proton affinities, and pKa values of a large series of edaravone derivatives: implication for their free radical scavenging activity. J Phys Chem B 115:10375–10384
Pérez-González A, Galano A, Ortiz JV (2014) Vertical ionization energies of free radicals and electron detachment energies of their anions: a comparison of direct and indirect methods versus experiment. J Phys Chem 118:6125–6131
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106:4049–4050
Hirshfeld F (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138
Eyring HJ (1935) The activated complex in chemical reactions. Chem Phys 3:107–115
Evans MG, Polanyi M (1935) Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans Faraday Soc 31:875–894
Truhlar DG, Hase WL, Hynes JT (1983) Current status of transition-state theory. J Phys Chem 87:2664–2682
Marcus RA (1965) Chemical and electrochemical electron-transfer theory. Annu Rev Phys Chem 16:155–196
Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Mod Phys 65:599–610
Marcus RA (1997) Transfer reactions in chemistry. Theory and experiment. Pure Appl Chem 69:13–30
Vargas-Sánchez RD, Mendoza-Wilson AM, Balandrán-Quintana RR, Torrescano-Urrutia GR, Sánchez-Escalante A (2015) Study of the molecular structure and chemical reactivity of pinocembrin by DFT calculations. Comp Theoret Chem 1058:21–27
Vargas-Sánchez RD, Mendoza-Wilson AM, Torrescano-Urrutia GR, Sánchez-Escalante A (2015) Antiradical potential of phenolic compounds fingerprints of propolis extracts: DFT approach. Comp Theoret Chem 1066:7–13
La Rocca MV, Rutkowski M, Ringeissen S, Gomar J, Frantz MC, Ngom S, Adamo C (2016) Benchmarking the DFT methodology for assessing antioxidant-related properties: quercetin and edaravone as case studies. J Mol Model 22:250
Marković Z, Đorović J, Dekić M, Radulović M, Marković S, Ilić M (2013). Chem Pap 67:1453
García-Hernández E, Garza J (2017) Reactivity sites in dopamine depend on its intramolecular hydrogen bond. J Mex Chem Soc 61:222–228
Kelly CP, Cramer CJ, Truhlar DG (2006) Adding explicit solvent molecules to continuum solvent calculations for the calculation of aqueous acid dissociation constants. J Phys Chem 110:2493–2499
Cramer CJ, Truhlar DG (2008) A universal approach to solvation modeling. Acc Chem Res 41:760–768
Pliego JR, Riveros JM (2000) New values for the absolute solvation free energy of univalent ions in aqueous solution. Chem Phys Lett 332:597–602
Pérez-González A, Galano A (2012) On the outstanding antioxidant capacity of edaravone derivatives through single electron transfer reactions. J Phys Chem B 116:1180–1188
Alberto ME, Russo N, Grand A, Galano A (2013) A physicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys Chem Chem Phys 15:4642–4650
Galano A (2015) Free radicals induced oxidative stress at a molecular level: the current status, challenges and perspectives of computational chemistry based protocols. J Mex Chem Soc 59:231–262
Vaidya V, Ingold KU, Pratt DA (2009) Garlic: source of the ultimate antioxidants-sulfenic acids. Angew Chem Int Ed 48:157–160
Acknowledgments
E. G.-H. acknowledges the computing time granted by LANCAD-CONACYT and TecNM (8338.20-PD) by the funding. The authors thank Laboratorio de Visualización y Cómputo en Paralelo at the UAM - Iztapalapa for the access to its computer facilities. A. P.-G. acknowledges the Program of Cátedras - CONACYT from CONACYT - UAMI (2015‑2025), ID-Investigador 435. E. C.-A. acknowledges the support given by the National Laboratory Supercomputing Southeast housed in the BUAP and by the high-performance computing system of PIDi-UTEM (SCC-PIDi-UTEM FONDEQUIP-EQM180180).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Pérez-González, A., García-Hernández, E. & Chigo-Anota, E. The antioxidant capacity of an imidazole alkaloids family through single-electron transfer reactions. J Mol Model 26, 321 (2020). https://doi.org/10.1007/s00894-020-04583-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04583-2