Abstract
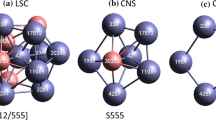
In this study, firstly favorable glass–forming composition for the binary Co–Ni alloy is identified as Co50Ni50 based on statistically evaluated thermodynamic parameters such as mixing enthalpy (∆Hmix), mixing entropy (∆Smix), and topological parameter such as atomic size difference (δ). Secondly, molecular dynamics (MD) simulations have been performed to investigate the glass–forming ability (GFA) and cluster evolution during the rapid solidification (7.67 K/ps) of Co50Ni50 under hydrostatic pressure (0, 0.25, 0.50, 1, 1.25, 2, 3, 5 GPa). It has been observed that with increasing pressure, glass transition temperature (Tg) also increases thereby increasing the GFA of Co50Ni50. Moreover, Voronoi cluster analysis reveals that quasi–icosahedral type clusters such as <0281> and <0282>, mixed types of cluster such as <0363>, <0364>, <1254>, and <0372> and crystal type clusters such as <0443> and <0444> have maximum population among the other clusters at different pressures at Co as well as Ni-centered atoms.


















Similar content being viewed by others
References
Lee HJ, Cagin T, Johnson WL, Goddard WA (2003) Criteria for formation of metallic glasses: the role of atomic size ratio. J. Chem. Phys. 119:9858–9870. https://doi.org/10.1063/1.1615494
Suryanarayana C, Inoue A (2011) Bulk metallic glasses. CRC Press, Boca Raton
Lai L, He R, Ding K, Liu T, Liu R, Chen Y, Guo S (2019) Ternary Co–Mo–B bulk metallic glasses with ultrahigh strength and good ductility. J. Non-Cryst. Solids 524:119657. https://doi.org/10.1016/j.jnoncrysol.2019.119657
Celtek M, Sengul S (2018) Thermodynamic and dynamical properties and structural evolution of binary Zr80Pt20 metallic liquids and glasses: molecular dynamics simulations. J. Non-Cryst. Solids 498:32–41. https://doi.org/10.1016/j.jnoncrysol.2018.06.003
Zhonga C, Cao QP, Wang XD, Zhang DX, Fecht HJ, Jiang JZ (2017) Relationship of deformation mode with strain–dependent shear transformation zone size in Cu–Zr metallic glasses using molecular dynamics simulations. J. Non-Cryst. Solids 469:45–50. https://doi.org/10.1016/j.jnoncrysol.2017.04.008
Mo J, Liu H, Zhang Y, Wang M, Zhang L, Liu B, Yang W (2017) Effects of pressure on structure and mechanical property in monatomic metallic glass. J. Non-Cryst. Solids 464:1–4. https://doi.org/10.1016/j.jnoncrysol.2017.03.013
Li Y, Zhao S, Liu Y, Gong P, Schroers J (2017) How many bulk metallic glasses are there? ACS Comb. Sci. 19:687–693. https://doi.org/10.1021/acscombsci.7b00048
Park ES, Chang HJ, Kyeong JS, Kim DH (2008) Role of minor addition of metallic alloying elements in formation and properties of Cu–Ti–rich bulk metallic glasses. J. Mater. Res. 23:1995–2002. https://doi.org/10.1557/JMR.2008.0246
Wang J, Li R, Hua N, Zhang T (2011) Co-based ternary bulk metallic glasses with ultrahigh strength and plasticity. J. Mater. Res. 26:2072–2079. https://doi.org/10.1557/jmr.2011.187
Chattopadhyay C, Satish Idury KSN, Bhatt J, Mondal K, Murty BS (2016) Critical evaluation of glass forming ability criteria. Mater. Sci.Technol 32:380–400. https://doi.org/10.1179/1743284715Y.0000000104
Zhang K, Wang M, Papanikolaou S, Liu Y, Schroers J, Shattuck MD, O'Hern CS (2013) Computational studies of the glass–forming ability of model bulk metallic glasses. J. Chem. Phys. 139:124503. https://doi.org/10.1063/1.4821637
Jiang JZ, Gerward L, Xu YS (2002) Pressure effect on crystallization kinetics in bulk glass. Appl. Phys. Lett. 81:4347–4349. https://doi.org/10.1063/1.1527227
Caris J, Lewandowski JJ (2010) Pressure effects on metallic glasses. Acta Mater. 58:1026–1036. https://doi.org/10.1016/j.actamat.2009.10.018
Vatamanu LO, Lewandowski JJ (2013) Pressure and temperature effects on tensile strength and plasticity of metallic glasses. Mech. Matter. 67:86–93. https://doi.org/10.1016/j.mechmat.2012.11.011
Liu LF, Yang J, Hu J, Li HQ, Guo SB (2013) Effect of hydrostatic pressure on shear banding behaviors in bulk metallic glasses. Mater. Lett. 93:289–292. https://doi.org/10.1016/j.matlet.2012.11.032
Feng SD, Jiao W, Jing Q, Qi L, Pan SP, Li G, Ma MZ, Wang WH, Liu RP (2016) Structural evolution of nanoscale metallic glasses during high–pressure torsion: a molecular dynamics analysis. Sci. Rep. 6:36627. https://doi.org/10.1038/srep36627
Miyazaki N, Wakeda M, Wang YJ, Ogata S (2016) Prediction of pressure–promoted thermal rejuvenation in metallic glasses. Npj Comp. Mater. 2:16013. https://doi.org/10.1038/npjcompumats.2016.13
Wang C, Yang ZZ, Ma T, Sun YT, Yin YY, Gong Y, Gu L, Wen P, Zhu PW, Long YW, Yu XH, Jin CQ, Wang WH, Bai HY (2017) High stored energy of metallic glasses induced by high pressure. Appl. Phys. Lett. 110:111901. https://doi.org/10.1063/1.4978600
Mishra S, Pal S (2018) Variation of glass transition temperature of Al90Sm10 metallic glass under pressurized cooling. J. Non-Cryst. Solids 500:249–259. https://doi.org/10.1016/j.jnoncrysol.2018.08.006
Zhou Z, Wang H, Li M (2019) Hydrostatic pressure effect on metallic glasses: a theoretical prediction. J. Appl. Phys. 126:145901. https://doi.org/10.1063/1.5118221
Andrew R L (2001) Molecular modeling principles and applications, 2nd, editor, Pearson Education Limited
Sun Y, Zhang Y, Zhang F, Ye Z, Ding Z, Wang CZ, Ho KM (2016) Cooling rate dependence of structural order in Al90Sm10 metallic glass. J. Appl. Phys. 120:015901. https://doi.org/10.1063/1.4955223
Hufnagel TC, Schuh CA, Falk ML (2016) Deformation of metallic glasses: recent developments in theory, simulations, and experiments. Acta Mater. 109:375–393. https://doi.org/10.1016/j.actamat.2016.01.049
Ding J, Ma E (2017) Computational modeling sheds light on structural evolution in metallic glasses and supercooled liquids. Npj Comput. Mater 9:1–12. https://doi.org/10.1038/s41524-017-0007-1
Zhong C, Zhang H, Cao QP, Wang XD, Zhang DX, Ramamurty U, Jiang JZ (2016) Deformation behavior of metallic glasses with shear band like atomic structure: a molecular dynamics study. Sci. Rep. 6:30935. https://doi.org/10.1038/srep30935
Bailey NP, Schiøtz J, Jacobsen KW (2004) Simulation of Cu–Mg metallic glass: thermodynamics and structure. Phys. Rev. B 69:144205. https://doi.org/10.1103/PhysRevB.69.144205
Wang XD, Yin S, Cao QP, Jiang JZ, Franz H, Jin ZH (2008) Atomic structure of binary Cu64.5Zr35.5 bulk metallic glass. Appl. Phys. Lett. 92:011902. https://doi.org/10.1063/1.2828694
Lu BF, Kong LT, Laws KJ, Xu WQ, Jiang Z, Huang YY, Ferry M, Li JF, Zhou YH (2018) EXAFS and molecular dynamics simulation studies of Cu–Zr metallic glass: short–to–medium range order and glass forming ability. Mater. Charact. 414:41–48. https://doi.org/10.1016/j.matchar.2018.04.036
Sheng HW, Ma E, Kramer MJ (2012) Relating dynamic properties to atomic structure in metallic glasses. JOM 64:856–881. https://doi.org/10.1007/s11837-012-0360-y
Gulenko A, Chung LF, Gao J, Todd I, Hannon AC, Martin RA, Christie JK (2017) Atomic structure of Mg–based metallic glasses from molecular dynamics and neutron diffraction. Phys. Chem. Chem. Phys. 19:8504–8515. https://doi.org/10.1039/C6CP03261C
Kumar V, Fujita T, Konno K, Matsuura M, Chen MW, Inoue A, Kawazoe Y (2011) Atomic and electronic structure of Pd40Ni40P20 bulk metallic glass from ab initio simulations. Phys. Rev B 84:134204. https://doi.org/10.1103/PhysRevB.84.134204
Srivastava AP, Das N, Sharma SK, Sinha AK, Srivastava D, Pujari PK, Dey GK (2016) Investigation of medium range order and glass forming ability of metallic glass Co69FexSi21−xB10 (x= 3, 5, and 7). J. Phys. D. Appl. Phys. 49:225303. https://doi.org/10.1088/0022-3727/49/22/225303
Mukhtar A, Mehmood T, Wu KM (2017) Investigation of phase transformation of CoNi alloy nanowires at high potential. IOP Conf. Ser Mater. Sci. Eng. 239:012017. https://doi.org/10.1088/1757-899X/239/1/012017
Zhang Y, Zhou YJ, Lin JP, Chen GL, Liaw PK (2008) Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 10:534–538. https://doi.org/10.1002/adem.200700240
Guo S, Liu CT (2011) Phase stability in high entropy alloys: formation of solid–solution phase or amorphous phase. Prog. Nat. Sci. Mater. Inter. 21:433–446. https://doi.org/10.1016/S1002-0071(12)60080-X
Tsai MH, Yeh JW (2014) High–entropy alloys: a critical review. Mater. Res. Lett. 2:107–123. https://doi.org/10.1080/21663831.2014.912690
Yang X, Zhang Y (2012) Prediction of high–entropy stabilized solid–solution in multi–component alloys. Mater. Chem. Phys. 132:233–238. https://doi.org/10.1016/j.matchemphys.2011.11.021
Guo S (2015) Phase selection rules for cast high entropy alloys: an overview. Mater. Sci. Technol. 31:1–8. https://doi.org/10.1179/1743284715Y.0000000018
Yurchenko N, Stepanov N, Salishchev G (2016) Laves–phase formation criterion for high–entropy alloys. Mater. Sci. Technol. 11:17–22. https://doi.org/10.1080/02670836.2016.1153277
Xing QW, Zhang Y (2017) Amorphous phase formation rules in high–entropy alloys. Chin. Phys. B 26:018104. https://doi.org/10.1088/1674-1056/26/1/018104
Zhang Y (2019) High–entropy materials: a brief introduction. Springer Nature Singapore Pte Ltd. https://doi.org/10.1007/978-981-13-8526-1
Nishizawa T, Ishida K (1983) The Co−Ni (cobalt–nickel) system. Bull. Alloy Phase Diagr. 4:390–395. https://doi.org/10.1007/BF02868090
Takeuchi A, Inoue A (2001) Quantitative evaluation of critical cooling rate for metallic glasses. Mater. Sci. Eng. A 304–306:446–451. https://doi.org/10.1016/S0921-5093(00)01446-5
Takeuchi A, Inoue A (2010) Mixing enthalpy of liquid phase calculated by Miedema’s scheme and approximated with sub–regular solution model for assessing forming ability of amorphous and glassy alloys. Intermetallics 18:1779–1789. https://doi.org/10.1016/j.intermet.2010.06.003
Plimpton S (1995) Fast parallel algorithms for short–range molecular dynamics. J. Comput. Phys. 117:1–19. https://doi.org/10.1006/jcph.1995.1039
Béland LK, Lu C, Osetskiy YN, Samolyuk GD, Caro A, Wang L, Stoller RE (2016) Features of primary damage by high energy displacement cascades in concentrated Ni–based alloys. J. Appl. Phys. 119:085901. https://doi.org/10.1063/1.4942533
Mishin Y (2004) Atomistic modeling of the γ and γ′–phases of the Ni–Al system. Acta Mater. 52:1451–1467. https://doi.org/10.1016/j.actamat.2003.11.026
Pun GPP, Mishin Y (2012) Embedded–atom potential for hcp and fcc cobalt. Phys. Rev. B 86:134116. https://doi.org/10.1103/PhysRevB.86.134116
Li F, Zhang H, Liu X, Yu C, Lu Z (2018) Effects of cooling rate on the atomic structure of Cu64Zr36 binary metallic glass. Comput. Mater. Sci. 141:59–67. https://doi.org/10.1016/j.commatsci.2017.09.026
Martyna GJ, Klein ML, Tuckerman M (1992) Nosé–Hoover chains: the canonical ensemble via continuous dynamics. J. Chem. Phys. 97:2635–2643. https://doi.org/10.1063/1.463940
Durandurdu M (2012) Ab initio modeling of metallic Pd80Si20 glass. Comput. Mater. Sci. 65:44–47. https://doi.org/10.1016/j.commatsci.2012.06.040
Finney JL (1970) Random packings and the structure of simple liquids I. The geometry of random close packing. Proc. R. Soc. A 319:479–493. https://doi.org/10.1098/rspa.1970.0189
Li F, Liu XJ, Lu ZP (2014) Atomic structural evolution during glass formation of a Cu–Zr binary metallic glass. Comput. Mater. Sci. 85:147–153. https://doi.org/10.1016/j.commatsci.2013.12.058
Stukowski A (2010) Visualization and analysis of atomistic simulation data with OVITO–the open visualization tool. Model. Simul. Mater. Sci. Eng. 18:015012. https://doi.org/10.1088/0965-0393/18/1/015012
Meraj MD, Pal S (2016) The effect of temperature on creep behaviour of porous (1 at.%) nano crystalline nickel. Trans. Indian Inst. Metals 69:277–282. https://doi.org/10.1007/s12666-015-0763-x
Park JM, Na JH, Kim DH, Kim KB, Mattern N, Kuhn U, Eckert J (2010) Medium range ordering and its effect on plasticity of Fe–Mn–B–Y–Nb bulk metallic glass. Philos. Mag. 90:2619–2633. https://doi.org/10.1080/14786431003662556
Mattern N (2007) Structure formation in liquid and amorphous metallic alloys. J. Non–Crys. Solids 353:1723–1731. https://doi.org/10.1016/j.jnoncrysol.2007.01.042
Fujita T, Konno K, Zhang W, Kumar V, Matsuura M, Inoue A, Sakurai T, Chen MW (2009) Atomic–scale heterogeneity of a multicomponent bulk metallic glass with excellent glass forming ability. Phys. Rev. Lett. 103:075502. https://doi.org/10.1103/PhysRevLett.103.075502
Greer AL (1993) Confusion by design. Nature 366:303–304. https://doi.org/10.1038/366303a0
Senkov ON, Miracle DB (2001) Effect of the atomic size distribution on glass forming ability of amorphous metallic alloys. MRS Bull. 36:2183–2198. https://doi.org/10.1016/S0025-5408(01)00715-2
Busch R (2000) The thermophysical properties of bulk metallic glass–forming liquids. JOM 52:39–42. https://doi.org/10.1007/s11837-000-0160-7
Jiang D, Wen D, Tian Z, Liu R (2016) Glass formation and cluster evolution in the rapidly solidified monatomic metallic liquid Ta under high pressure. Phys. A: Stat. Mech. Appl. 463:174–181. https://doi.org/10.1016/j.physa.2016.07.032
Zhang H, Mo Y, Tian Z, Liu R, Zhou L, Hou Z (2017) The effect of pressure on the crystallization of rapidly supercooled zirconium melts. Phys. Chem. Chem. Phys. 19:12310–12320. https://doi.org/10.1039/C7CP00865A
Sanchez IC (1974) Towards a theory of viscosity for glass-forming liquids. J. Appl. Phys. 45:4204–4215. https://doi.org/10.1063/1.1663037
Jiang JZ, Roseker W, Sikorski M, Cao QP, Xu F (2004) Pressure effect of glass transition temperature in Zr46.8Ti8.2Cu7.5Ni10Be27.5 bulk metallic glass. Appl. Phys. Lett. 84:1871. https://doi.org/10.1063/1.1675937
Samwer K, Busch R, Johnson WL (1999) Change of compressibility at the glass transition and Prigogine–Defay ratio in ZrTiCuNiBe alloys. Phys. Rev. Lett. 82:580–583. https://doi.org/10.1103/PhysRevLett.82.580
Jia Z, Duan X, Qin P, Zhang W, Wang W, Yang C, Sun H, Wang S, Zhang LC (2017) Disordered atomic packing structure of metallic glass: toward ultrafast hydroxyl radicals production rate and strong electron transfer ability in catalytic performance. Adv. Funct. Mater. 27:1702258. https://doi.org/10.1002/adfm.201702258
Feng SD, Chan KC, Chen SH, Zhao L, Liu RP (2017) The role of configurational disorder on plastic and dynamic deformation in Cu64Zr36 metallic glasses: a molecular dynamics analysis. Sci. Rep. 7:40969. https://doi.org/10.1038/srep40969
Turnbull D (1969) Under what conditions can a glass be formed? Contemp. Phys. 10:73–488. https://doi.org/10.1080/00107516908204405
Srivastava AP, Srivastava D, Sudarshan K, Sharma SK, Pujari PK, Majumdar B, Suresh KG, Dey GK (2012) Correlation of soft magnetic properties with free volume and medium range ordering in metallic glasses probed by fluctuation microscopy and positron annihilation technique. J. Magn. Magn. Mater. 324:2476–2482. https://doi.org/10.1016/j.jmmm.2012.03.014
Wen J, Cheng YQ, Wang JQ, Ma E (2009) Distinguishing medium–range order in metallic glasses using fluctuation electron microscopy: a theoretical study using atomic models. J. Appl. Phys. 105:043519. https://doi.org/10.1063/1.3079514
Colín JG, Valladares AA, Valladares RM, Valladare A (2015) Short–range order in ab initio computer generated amorphous and liquid Cu–Zr alloys: a new approach. Physica B: Cond. Matter. 475:140–147. https://doi.org/10.1016/j.physb.2015.07.027
Wu ZW, Li MZ, Wang WH, Liu KX (2015) Hidden topological order and its correlation with glass–forming ability in metallic glasses. Nat. Commun. 6:7035. https://doi.org/10.1038/ncomms7035
Miracle DB, Lord EA, Ranganathan S (2006) Candidate atomic cluster configurations in metallic glass structures. Mater. Trans. 47:1737–1742. https://doi.org/10.2320/matertrans.47.1737
Sheng HW, Luo WK, Alamgir FM, Bai JM, Ma E (2006) Atomic packing and short–to–medium–range order in metallic glasses. Nature 439:419–425. https://doi.org/10.1038/nature04421
Kbirou M, Trady S, Hasnaoui A, Mazroui M (2018) Short and medium–range orders in Co3Al metallic glass. Chem. Phys. 513:58–66. https://doi.org/10.1016/j.chemphys.2018.06.018
Frank FC, Kasper JS (1958) Complex alloy structures regarded as sphere packings. I. Definitions and basic principles. Acta Crystallogr. 11:184–190. https://doi.org/10.1107/S0365110X58000487
Hwang J (2011) Nanometer scale atomic structure of zirconium based bulk, Ph.D. diss., Te University of Wisconsin–Madiscon. http://digital.library.wisc.edu/1793/63366
Sun YL, Shen J, Valladares AA (2009) Atomic structure and diffusion in Cu60Zr40 metallic liquid and glass: molecular dynamics simulations. J. Appl. Phys. 106:073520. https://doi.org/10.1063/1.3245324
Toninelli C, Wyart M, Berthier L, Biroli G, Bouchaud JP (2005) Dynamical susceptibility of glass formers: contrasting the predictions of theoretical scenarios. Phys. Rev. E 71:041505. https://doi.org/10.1103/PhysRevE.71.041505
Karmakara S, Dasgupta C, Sastry S (2009) Growing length and time scales in glass–forming liquids. PNAS 106:3675–3679. https://doi.org/10.1073/pnas.0811082106
Kluge M, Schober HR (2004) Diffusion and jump–length distribution in liquid and amorphous Cu33Zr67. Phys. Rev. B 70:224209. https://doi.org/10.1103/PhysRevB.70.224209
Wong K, Kana HW, Mole R, Yu D, Chathotha SM (2018) The influence of short–range structures on atomic caging in glass–forming Cu–Zr–Al melts. Intermetallics 102:114–119. https://doi.org/10.1016/j.intermet.2018.09.009
Mendelev MI, Kramer MJ, Ott RT, Sordelet DJ (2009) Molecular dynamics simulation of diffusion in supercooled Cu–Zr alloys. Philos. Mag. 89:109–126. https://doi.org/10.1080/14786430802570648
Faupel F, Frank W, Macht MP, Mehrer H, Naundorf V, Ratzke K, Schober H, Sharma S, Teichler H (2003) Diffusion in metallic glasses and supercooled melts. Rev. Mod. Phys. 75:237–280. https://doi.org/10.1103/RevModPhys.75.237
Liu XJ, Wang SD, Fan HY, Ye YF, Wang H, Wu Y, Lu ZP (2018) Static atomic–scale structural heterogeneity and its effects on glass formation and dynamics of metallic glasses. Intermetallics 101:133–143. https://doi.org/10.1016/j.intermet.2018.08.001
Jaiswal A, Egami T, Zhang Y (2015) Atomic–scale dynamics of a model glass–forming metallic liquid: dynamical crossover, dynamical decoupling, and dynamical clustering. Phys. Rev. B 91:134204. https://doi.org/10.1103/PhysRevB.91.134204
Jiang JZ, Gerward L, Xu YS (2002) Pressure effect on crystallization kinetics in Zr46.8Ti8.2Cu7.5Ni10Be27.5 bulk glass. Appl. Phys. Lett. 81:4347. https://doi.org/10.1063/1.1527227
Acknowledgments
Authors are thankful to the Department of Metallurgical and Materials Engineering, National Institute of Technology Rourkela for providing the high-performance computational facilities to carry out these computational simulations.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2146 kb)
Rights and permissions
About this article
Cite this article
Deshmukh, A.A., Pal, S. Dynamic probing of structural evolution for Co50Ni50 metallic glass during pressurized cooling using atomistic simulation. J Mol Model 26, 208 (2020). https://doi.org/10.1007/s00894-020-04468-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04468-4