Abstract
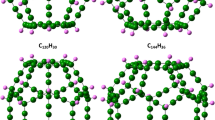
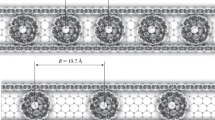
In this paper, the structural and electronic properties of a CN fullerene with N = 20, 60, 80, 180, and 240 have been investigated using a sp3 tight-binding model. The analytical expressions for the calculation of the total number of carbon atoms, hexagons, pentagons, and bonds found within the geometrical structure of a CN fullerene have been developed and verified using the simulation, therefore proving the validation of both the simulation and analytical results. The simulation results show that the total number of carbon atoms within fullerene is equal to the value of N and the total number of hexagons, pentagons, and bonds within the structure of a fullerene increases with the increase in the value of N. Further, the electronic properties of these fullerenes have been identified with the help of their energy level diagrams obtained using the simulation. It has been observed that the C20 and C80 fullerenes are metallic because of their zero band gaps while the C60 fullerene is an insulator with a very wide band gap of 5 eV whereas the C180 and C240 fullerenes are semiconducting with band gaps of 1.43 eV and 1.05 eV, respectively. Finally, it has been observed from these studies that the metallic fullerenes are best suited for interconnects and the semiconducting fullerenes are bested suited as a channel material for designing high-performance nanoelectronic devices.


Similar content being viewed by others
References
Haddon RC, Perel AS, Morris RC, Palstra TTM, Hebard AF, Fleming RM (1995) C60 thin film transistors. Appl Phys Lett 67:121–123
Haddon RC (1996) C70 thin film transistors. J Am Chem Soc 118:3041–3042
Kobayashi S, Takenobu T, Mori S, Fujiwara A, Iwasa Y (2003) Fabrication and characterization of C60 thin-film transistors with high field-effect mobility. Appl Phys Lett 82:4581–4583
Kanbara T, Shibata K, Fujiki S, Kubozono Y, Kashino S, Urisu T, Sakai M, Fujiwara A, Kumashiro R, Tanigaki K (2003) N-channel field effect transistors with fullerene thin films and their application to a logic gate circuit. Chem Phys Lett 379:223–229
Kobayashi S, Mori S, Iida S, Ando H, Takenobu T, Taguchi Y, Fujiwara A, Taninaka A, Shinohara H, Iwasa Y (2003) Conductivity and field effect transistor of La2@C80 metallofullerene. J Am Chem Soc 125:8116–8117
Shibata K, Kubozono Y, Kanbara T, Hosokawa T, Fujiwara A, Ito Y, Shinohara H (2004) Fabrication and characteristics of C84 fullerene field-effect transistors. Appl Phys Lett 84:2572–2574
Vasconcelos RC, Aleixo VFP, Nero JD (2017) Organic field effect transistor composed by fullerene C60 and heterojunctions. Physica E 86:142–145
Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O'Malley KL (2001) Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord 7:243–246
Goldshleger NF (2001) Fullerenes and fullerene-based materials in catalysis. Fuller Sci Technol 9:255–280
Harneit W (2002) Fullerene-based electron-spin quantum computer. Phys Rev A 65:032322
Illescas BM, Martín N (2006) Fullerene-based electron acceptors. Comptes Rendus Chimie 9:1038–1050
Sharma M, Bhatiaa R, Gupta V, Chand S, Raghunathan P, Eswarana SV (2011) Soluble functionalised fullerenes for photovoltaics. Synth Met 161:844
García M, Guadarrama P, Ramos E, Fomine S (2011) Rectifying behavior of [60]fullerene charge transfer complexes: a theoretical study. Synth Met 161:2390–2396
Guo F, Xiao Z, Huang J (2013) Fullerene photodetectors with a linear dynamic range of 90 db enabled by a cross-linkable buffer layer. Adv Opt Mater 1:289–294
Dellinger A, Zhou Z, Connor J, Madhankumar AB, Pamujula S, Sayes CM, Kepley CL (2013) Application of fullerenes in nanomedicine: An update. Nanomedicine 8:1191–1208
Pilehvar S, Wael KD (2015) Recent advances in electrochemical biosensors based on fullerene-C60 nano-structured platforms. Biosensors 5:712–735
Lanzellotto C, Favero G, Antonelli ML, Tortolini C, Cannistraro S, Coppari E, Mazzei F (2014) Nanostructured enzymatic biosensor based on fullerene and gold nanoparticles: preparation, characterization and analytical applications. Biosens Bioelectron 55:430–437
Kuzmany H, Winter J, Burger B (1997) Polymeric fullerenes. Synth Met 85:1173–1177
Ko YG, Hahm SG, Murata K, Kim YY, Ree BJ, Song S, Michinobu T, Ree M (2014) New fullerene-based polymers and their electrical memory characteristics. Macromolecules 47:8154–8163
Li Z, Wong HC, Huang Z, Zhong H, Tan CH, Tsoi WC, Kim JS, Durrant JR, Cabral JT (2013). Nat Commun 4:2227
Mohajeri A, Omidvar A (2015) Fullerene-based materials for solar cell applications: design of novel acceptors for efficient polymer solar cells – a DFT study. Phys Chem Chem Phys 17:22367
Zhang F, Inganäs O, Zhou Y, Vandewal K (2016) Development of polymer–fullerene solar cells. Natl Sci Rev 3:222–239
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60:Buckmister fullerene. Nature 318:162–163
Prinzbach H, Weiler A, Landenberger P, Wahl F, Wörth J, Scott LT, Gelmont M, Olevano D, Issendorff BV (2000) Gasphase production and photoelectron spectroscopy of the smallest fullerene, C20. Nature 407:60–63
Sokolova S, Lüchow A, Anderson JB (2000) Energetics of carbon clusters C20 from all-electron quantum Monte Carlo calculations. Chem Phys Lett 323:229–233
Spagnolatti I, Bernasconi M, Benedek G (2002) Electron-phonon interaction in the solid form of the smallest fullerene C20. Europhys Lett 59:572–578
An YP, Yang CL, Wang MS, Ma XG, Wang DH (2009) First principles study of structure and quantum transport properties of C20 fullerene. J Chem Phys 131:024311
An YP, Yang CL, Wang MS, Ma XG, Wang DH (2010) First principles study of transport properties of endohedral Li@C20 metallofullerene. Curr Appl Phys 10:260–265
Kroto HW (1987) The stability of the fullerene Cn, with 24, 28, 32, 36, 50, 60, and 70. Nature 329:529–531
Rabenau T, Simon A, Kremer RK, Sohmen E (1993) The energy gaps of fullerene C6o and C70 determined from the temperature dependent microwave conductivity. Z Phys B 90:69–72
Zhang RQ, Feng YQ, Lee ST, Bai CL (2004) Electrical transport and electronic delocalization of small fullerenes. J Phys Chem B 108:16636
Lee SM, Nicholls RJ, Nguyen-Manh D, Pettifor DG, Briggs GAD, Lazar S, Pankhurst DA, Cockayne DJH (2005) Electron energy loss spectra of C60 and C70 fullerenes. Chem Phys Lett 404:206–211
Małolepsza E, Witek HA (2007) Comparison of geometric, electronic, and vibrational properties for isomers of small fullerenes C20-C36. J Phys Chem A 111:6649–6657
Kaur M, Sawhney RS, Engles D (2017) Ab-initio molecular characterization of nonclassical fullerenes cluster using two probe approach. J Mater Res 32:414–425
Dass D (2018) Structural analysis, electronic properties, and band gaps of a graphene nanoribbon: a new 2D materials. Superlattice Microst 115:88–107
Dass D (2018) Structural parameters, electronic properties, and band gaps of a single walled carbon nanotube: a pz orbital tight binding study. Superlattice Microst 120:108–126
Wang BC, Chiu YN (1993) Geometry and orbital symmetry relationships of giant and hyperfullerenes: C240, C540, C960 and C1500. Synth Met 56:2949–2954
Forro L, Mihaly L (2001) Electronic properties of doped fullerenes. Rep Prog Phys 64:649–699
Cho K (2015) MSL Simulator
Harrison WA (1999) World Scientific Publishing, Singapore
Menon M, Subbaswamy KR (1991) Universal parameter tight-binding molecular dynamics: application to C60. Phys Rev Lett 67:3487–3490
Menon M, Subbaswamy KR (1991) Transferable nonorthogonal tight-binding scheme for silicon. Phys Rev B 50:11577
Menon M (1998) A transferable nonorthogonal tight-binding scheme for germanium. J Phys Condens Matter 10:10991
Vogl P, Hjalmarsons HP, Dow JD (1983) A semi-empirical tight-binding theory of the electronic structure of semiconductors. J Phys Chem Solids 44:365–378
Tomanek D, Schluter MA (1987) Structure and bonding of small semiconductor clusters. Phys Rev B 36:1208–1217
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dass, D. Structural and electronic properties of a CN fullerene with N = 20, 60, 80, 180, and 240. J Mol Model 26, 9 (2020). https://doi.org/10.1007/s00894-019-4207-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4207-0