Abstract
The present study provides a detailed quantum chemical description of the physicochemical interactions between poly-vinylidene fluoride (PVDF) and 1-butyl-3-methyl-imidazolium tetrafluoro borate ([BMIM][BF4]) ionic liquid (IL). Geometry optimization and frequency calculations are carried out for four monomer units of α- and β-PVDF, [BMIM][BF4], and PVDF/[BMIM][BF4] using dispersion corrected density functional theory. The effects of solvation on the systems under study are demonstrated for three polar aprotic solvents, namely tetra-hydrofuran (THF), acetone, and n,n-dimethyl formamide (DMF) using the integral equation formalism polarizable continuum model (IEFPCM). Calculated negative solvation free energy values suggest solution phase stability of the systems under study. Binding and interaction energies for β-PVDF/IL are found higher in magnitude than those for α-PVDF/IL. The nonbonding interaction phenomenon of β-PVDF/[BMIM][BF4] is elucidated on the basis of natural bond orbital (NBO), Bader’s quantum theory of atoms in molecules (QTAIM), delocalization indices, Hirshfeld surface, and reduced density gradient (RDG) analyses. Both anions and cations of ionic liquids are found to show weak van der Waals interaction with PVDF molecule but the anion ([BF4]−)/PVDF interaction is found to be stronger than cation ([BMIM]+)/PVDF interaction. Inter-unit C−H⋯F type hydrogen bonds are found to show improper (causing blue shifts in vibrational frequencies) nature. Frontier molecular orbital analysis is carried out, and different chemical parameters like electronegativity, chemical potential, chemical hardness and softness, and electrophilicity index are calculated using Koopmans’ theorem. Thermochemical calculations are also performed, and the variation in different standard thermodynamic parameters with temperature is formulated.

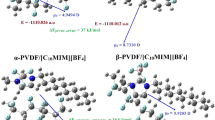
(a) Hirshfeld surface mapped onto electron density and (b) NCI isosurfaces showing inter-unit interactions of β-PVDF/[BMIM][BF4]















Similar content being viewed by others
References
Lines ME, Glass AM (1977) Principles and applications of ferroelectrics and related materials. Clarendon, Oxford
Itoh A, Takahashi Y, Furukawa T, Yajima H (2014) Solid-state calculations of poly(vinylidene fluoride) using the hybrid DFT method: spontaneous polarization of polymorphs. Polym J 46:207–211
Bohlé M, Bolton K (2014) Conformational studies of poly(vinylidene fluoride), poly(trifluoroethylene) and poly(vinylidene fluoride-co-trifluoroethylene) using density functional theory. Phys Chem Chem Phys 16:12929–12939
Nabata Y (1990) Structure of crosslinked poly (vinylidene fluoride) crystallized from melt under uniaxial compression. Jpn J Appl Phys 29:1298–1303
Gomes J, Nunes JS, Sencadas V, Lanceros-Mendez S (2010) Influence of the β-phase content and degree of crystallinity on the piezo-and ferroelectric properties of poly(vinylidene fluoride). Smart Mater Struct 19:065010 1–065010 7
Qian X, Wu S, Furman E, Zhang Q, Su J (2015) Ferroelectric polymers as multifunctional electroactive materials: recent advances, potential, and challenges. MRS Commun 5(2):115–129
Abolhasani MM, Zarejousheghani F, Cheng ZX, Naebe M (2015) A facile method to enhance ferroelectric properties in PVDF nanocomposites. RSC Adv 5:22471–22479
Mofokeng TG, Luyt AS, Pavlovic VP, Pavlovic VB, Dudic D, Vlahovic B, Djokovic V (2014) Ferroelectric nanocomposites of polyvinylidene fluoride/polymethyl methacrylate blend and BaTiO3 particles: fabrication of β-crystal polymorph rich matrix through mechanical activation of the filler. J Appl Phys 115:084109 1-9
Mahdi RI, Gan WC, Halim NA, Velayutham TS, Majid WHA (2015) Ferroelectric and pyroelectric properties of novel lead-free polyvinylidenefluoride-trifluoroethylene-Bi0.5Na0.5TiO3 nanocomposite thin films for sensing applications. Ceram Int 41:13836–13843
Zeng H, Sabirianov R, Mryasov O, Yan ML, Cho K, Sellmyer DJ (2002) Curie temperature of FePt : B2O3 nanocomposite films. Phys Rev B 127:1–6
Lee WG, Park BE, Park KE (2013) Ferroelectric properties of the organic films of poly(vinylidene fluoride-trifluoroethylene) blended with inorganic Pb(Zr, Ti)O3. Thin Solid Films 546:171–175
Xia W, Xu Z, Wen F, Zhang Z (2012) Electrical energy density and dielectric properties of poly(vinylidene fluoride-chlorotrifluoroethylene)/BaSrTiO3 nanocomposites. Ceram Int 38:1071–1075
Chan HLW, Chan WK, Zhang Y, Choy CL (1998) Pyroelectric and piezoelectric properties of lead titanate/polyvinylidene fluoride-trifluoroethylene 0-3 composites. IEEE Trans Dielectr Electr Insul 5:505–512
Fang M, Wang Z, Li H, Wen Y (2015) Fabrication and dielectric properties of Ba(Fe0.5Nb0.5)O3/poly(vinylidene fluoride) composites. Ceram Int 117:1–6
Xing C, You J, Li Y, Li J (2015) Nanostructured poly(vinylidene fluoride)/ionic liquid composites: formation of organic conductive nanodomains in polymer matrix. J Phys Chem C 119:21155–21164
Dias JC, Lopes AC, Magalhães B, Botelho G, Silva MM, Esperança JMSS, Lanceros-Mendez S (2015) High performance electromechanical actuators based on ionic liquid/poly(vinylidene fluoride). Polym Test 48:199–205
Mejri R, Dias JC, Lopes AC, Hentati SB, Silva MM, Botelho G, Mão de Ferro A, Esperança JMSS, Maceiras A, Laza JM, Vilas JL, León LM, Lanceros-Mendez S (2015) Effect of anion type in the performance of ionic liquid/poly(vinylidene fluoride) electromechaical actuators. Eur Polym J 71:304–313
Wang F, Lack A, Xie Z, Frübing P, Taubert A, Gerhard R (2012) Ionic-liquid-induced ferroelectric polarization in poly(vinylidene fluoride) thin films. Appl Phys Lett 100:1–6
Liang CL, Mai ZH, Xie Q, Bao RY, Yang W, Xie BH, Yang MB (2014) Induced formation of dominating polar phases of poly(vinylidene fluoride): positive ion−CF2 dipole or negative ion−CH2 dipole interaction. J Phys Chem B 118:9104–9111
Grimme S (2011) Density functional theory with London dispersion correction. WIREs Comput Mol Sci 1:211–228
Clark T, Koch R (1999) The chemist’s electronic book of orbitals. Springer, Heidelberg
Sarkar R, Kundu TK (2018) Density functional theory studies on PVDF/ionic liquid composite systems. J Chem Sci 130:115
Levine IN (2012) Quantum chemistry, 7th edn. Pearson, New York
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Garcia-Revilla M, Fransisco E, Popelier PLA, Pendas AM (2013) Domain-averaged exchange-correlation energies as a physical underpinning for chemical graphs. ChemPhysChem 14:1211–1218
Spackman MA, Byrom PG (1997) A novel definition of a molecule in a crystal. Chem Phys Lett 267:215–220
C-García J, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632
Ma W, Zhang J, Wang X (2008) Formation of poly(vinylidene fluoride) crystalline phases from tetrahydrofuran/N,N-dimethyl formamide mixed solvent. J Mater Sci 43:398–401
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum model solvation. 1. General formalism. J Chem Phys 132:114110 1-15
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson GJ, Fox DJ (2009) Gaussian 09, revision a.01. Gaussian Inc, Wallingford
Dennington R D, Ketith T A, Millam J M (2008) GaussView 5.0.8. Gaussian Inc, Wallingford
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Pal S, Kundu TK (2013) DFT-based inhibitor and promoter selection criteria for pentagonal dodecahedron methane hydrate cage. J Chem Sci 125:1259–1266
Řezáč J, Hobza P (2016) Benchmark calculations of interaction energies in noncovalent complexes and their applications. Chem Rev 116:5038–5071
Duijneveldt FBV, Duijneveldt-van JGCMV, Lenthe JHV (1994) State of the art in counterpoise theory. Chem Rev 94:1873–1885
Jeziorski B, Moszynski R, Szalewicz K (1994) Perturbation theory approach to intermolecular potential energy surfaces of van der Waals complexes. Chem Rev 94:1837–1930
Parrish RM, Burns LA, Smith DGA, Simmonett AC, DePrince AE, Hohenstein EG, Bozkaya U, Sokolov AY, Di Remigio R, Richard RM, Gonthier JF, James AM, McAlexander HR, Kumar A, Saitow M, Wang X, Pritchard BP, Verma P, Schaefer HF, Patkowski K, King RA, Valeev EF, Evangelista FA, Turney JM, Crawford TD, Sherrill CD (2017) Psi4 1.1: an open-source electronic structure program emphasizing automation, advanced libraries, and interoperability. J Chem Theory Comput 13(7):3185–3197
Ho J, Erton MZ (2016) Calculating free energy changes in continuum solvation models. J Phys Chem B 120:1319–1329
Solymar L, Walsh D, Syms RRA (2014) Electronic properties of materials, 9th edn. Oxford University Press, Oxford
Zhan CG, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107(20):4184–4195
Tsuneda T, Song JW, Suzuki S, Hirao K (2010) On Koopmans’ theorem in density functional theory. J Chem Phys 133:174101–174109
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Pal S, Kundu TK (2013) Stability analysis and frontier orbital study of different glycol and water complex. ISRN Phys Chem 2013:753139
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Guillaumes L, Salvador P, Simon S (2014) A fuzzy-atom analysis of electron delocalization on hydrogen bonds. J Phys Chem A 118:1142–1149
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Elyukhin VA (2016) Statistical thermodynamics of semiconductor alloys. Elsevier, Waltham
Irikura KK (2002) Thermo. Pl. National Institute of Standards and Technology, Gaithersburg, MD
Hasegawa R, Takahashi Y, Chatani Y, Tadokoro H (1971) Crystal structures of three crystalline forms of poly(vinylidene fluoride). Polym J 3:600–610
Wang ZY, Fan HQ, Su KH, Wen ZY (2006) Structure and piezoelectric properties of poly(vinylidene fluoride) studied by density functional theory. Polym J 47:7988–7996
Wu C, Visscher AD, Gates ID (2018) Interactions of biodegradable ionic liquids with a model napthenic acid. Nat Sci Rep 8:176
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Bahadur I, Kgomotso M, Ebenso EE, Redhi G (2016) Influence of temperature on molecular interactions of imidazolium-based ionic liquids with acetophenone: thermodynamic properties and quantum chemical studies. RSC Adv 6:104708–104723
Andersson MP, Uvdal P (2005) New scale factors for harmonic vibrational frequencies using the B3LYP density functional method with the triple-ζ basis set 6-311+G(d,p). J Phys Chem A 109:2937–2941
Ramer NJ, Marrone T, Stiso KA (2006) Structure and vibrational frequency determination for α-poly(vinylidene fluoride) using density-functional theory. Polym J 47:7160–7165
Katsyuba SA, Zvereva EE, Vidis A, Dyson PJ (2007) Application of density functional theory and vibrational spectroscopy toward the rational design of ionic liquids. J Phys Chem A 111:352–370
Shalu CSK, Singh RK, Chandra S (2003) Thermal stability, complexing behavior, and ionic transport of polymeric gel membranes based on polymer PVdF-HFP and ionic liquid, [BMIM][BF4]. J Phys Chem B 117:897–906
Nalwa HS (1995) Ferroelectric polymers: chemistry, physics and applications. Dekker, New York
Jeon Y, Sung J, Seo C, Lim H, Cheong H, Kang M, Moon B, Ouchi Y, Kim D (2008) Structures of ionic liquids with different anions studied by infrared vibration spectroscopy. J Phys Chem B 112:4735–4740
Cammi R, Cappelli C, Corni S, Tomasi J (2000) On the calculation of infrared intensities in solution within the polarizable continuum. J Phys Chem A 104:9874–9879
Yuan C, Yu H, Jia M, Su P, Luo Z, Yao J (2016) A theoretical study of weak interactions in phenylenediamine homodimer clusters. Phys Chem Chem Phys 18:29249–29257
Kerelson M, Zerner MC (1990) On the n-π* blue shift accompanying solvation. J Am Chem Soc 112:9405–9406
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 10:1527–1536
Olmo L, Morera-Boado C, Lopez R, Garcia de La Vega JM (2014) Electron density analysis of 1-butyl-3-methylimidazolium chloride ionic liquid. J Mol Model 20:2175 1–10
Venkataraman NS, Suvitha A, Kawazoe Y (2017) Intermolecular interaction in nucleobases and dimethyl sulfoxide/water molecules: a DFT, NBO, AIM and NCI analysis. J Mol Graph Model 78:48–60
Yoosefian M, Etminan N (2016) The role of solvent polarity in electronic properties, stability and reactive trend of a tryptophane/Pd doped SWCNT novel nanobiosensor from polar protic to non-polar solvents. RSC Adv 6:64818–64825
Jablonski M (2018) Bond paths between distant atoms do not necessarily indicate dominant interactions. J Comput Chem 39:2183–2195
Foroutan-Nejad C, Shahbazian S, Marek R (2018) Toward a consistent interpretation of the QTAIM: tortuous link between chemical bonds, interactions, and bond/line paths. Chemistry 20:10140–10152
Garcia-Revilla M, Popelier PLA, Fransisco E, Pendas AM (2011) Nature of chemical interactions from the profiles of electron delocalization indices. J Chem Theory Comput 7:1704–1711
Mayer I, Salvador P (2009) Effective atomic orbitals for fuzzy atoms. J Chem Phys 130:234106
Jablonski M, Sadlej AJ (2007) Blue-shifting intermolecular C−H⋯O interactions. J Phys Chem A 111:3423–3431
Hobza P, Havlas Z (2002) Improper, blue-shifting hydrogen bond. Theor Chem Accounts 108:325–334
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4263
Hobza P, Spirko V (1998) Anti-hydrogen bond in the benzene dimer and other carbon proton donor complexes. J Phys Chem A 102(15):2501–2504
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17901 kb)
Rights and permissions
About this article
Cite this article
Sarkar, R., Kundu, T.K. Nonbonding interaction analyses on PVDF/[BMIM][BF4] complex system in gas and solution phase. J Mol Model 25, 131 (2019). https://doi.org/10.1007/s00894-019-4020-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4020-9