Abstract
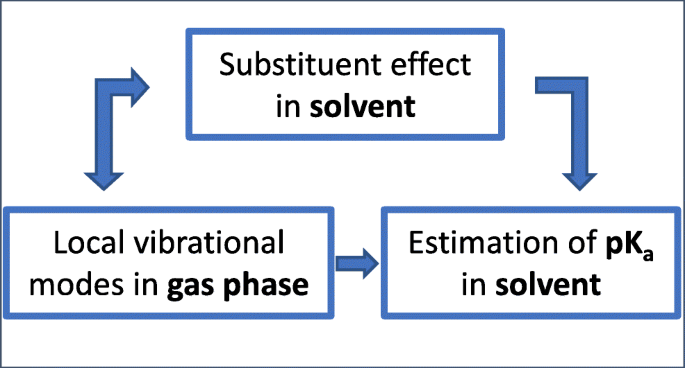
Molecular acidity is an important physicochemical property, which is often represented by the pKa value as the measure of acidity strength. However, the accurate calculation and prediction of pKa values is still an unsolved problem for computational chemistry. In this work, we present for the first time a direct correlation between pKa values and local vibrational frequencies for 15 different groups of compounds with various substituents. This correlation was derived from a quadratic function of two selected local vibrational frequencies as independent variables used to characterize electronic structure features influencing the molecular acidity. In total, 180 molecules were investigated with this correlation model. For each group of molecules, we found a strong correlation with root mean squared errors and mean absolute errors of less than 0.11 and 0.09 pKa units, respectively. The correlation between pKa and local vibrational modes, established in this work, can be generally applied to all compounds whose pKa values are dominated by electronic substituent effects. In this regard, the new correlation model constitutes a powerful link between the well-known Hammett equation and vibrational spectroscopy. Furthermore, it allows a quick prediction of the pKa values for new group members with different substituents.
pKa estimation via vibrational spectroscopy





Similar content being viewed by others
References
Cao X, Rong C, Zhong A, Tian Liu S (2018) Molecular acidity: an accurate description with information-theoretic approach in density functional reactivity theory. J Comp Chem 39:117–129
Cramer CJ, Truhlar DG (1999) Implicit solvation models: Equilibria, structure, spectra, and dynamics. Chem Rev 99(8):2161–2200
Alongi KS, Shields GC (2010) Theoretical calculations of acid dissociation constants: A review article. Annu Rep Comput Chem 6:113–138
Sastre S, Casasnovas R, Munozac F, Frau J (2016) Isodesmic reaction for accurate theoretical Ka calculations of amino acids and peptides. Phys Chem Chem Phys 18:11202–11212
Casasnovas R, Ortega-Castro J, Frau J, Donoso J, Munoz F (2014) Theoretical pKa calculations with continuum model solvents, alternative protocols to thermodynamic cycles. Int J Quantum Chem 114(20):1350–1363
Lanevskij K, Japertas P, Didziapetris R, Petrauskas A (2009) Ionization-specific prediction of blood–brain permeability. J Pharm Sci 98(1):122–134
Dreanic MP, Edge CM, Tuttle T (2017) New insights into the catalytic mechanism of aldose reductase: a QM/MM study. ACS Omega 2:5737–5747
Rajapakse HA, Nantermet PG, Selnick HG, Barrow JC, McGaughey GB, Munshi S, Lindsley SR, Young MB, Ngo PL, Holloway MK, Lai MT, Espeseth AS, Shi XP, Colussi D, Pietrak B, Crouthamel MC, Tugusheva K, Huang Q, Xu M, Simon AJ, Kuo L, Hazuda DJ, Graham S, Vacca JP (2010) Sar of tertiary carbinamine derived bace1 inhibitors: role of aspartate ligand amine pKa in enzyme inhibition. Bioorganic Med Chem Lett 20(6):1885–1889
Zhang J, Liu Z, Lian P, Qian J, Li X, Wang L, Fu W, Chen L, Wei X, Li C (2016) Selective imaging and cancer cell death via pH-switchable near-infrared fluorescence and photothermal effects. Chem Sci 7(9):5995–6005
Aiken GR, Hsu-Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45(8):3196–3201
Weng L, Temminghoff EJM, Lofts S, Tipping E, Van Riemsdijk WH (2002) Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ Sci Technol 36(22):4804–4810
Rao B, Simpson C, Lin H, Liang L, Gu B (2014) Determination of thiol functional groups on bacteria and natural organic matter in environmental systems. Talanta 119:240–247
Cheng J, Sprik M (2010) Acidity of the aqueous rutile TiO2(110) surface from density functional theory-based molecular dynamics. J Chem Theory Comput 6(3):880–889
Bryantsev VS (2013) Predicting the stability of aprotic solvents in Li-Air batteries: pKa calculations of aliphatic C-H acids in dimethyl sulfoxide. Chem Phys Lett 558:42–47. supplement C
Gallus DR, Wagner R, Wiemers-Meyer S, Winter M, Cekic-Laskovic I (2015) New in-sights into the structure-property relationship of high-voltage electrolyte components for lithium-ion batteries using the pKa value. Electrochimica Acta 184:410–416. supplement C
Lau YH, Clegg JK, Price JR, Macquart RB, Todd MH, Rutledge PJ (2018) Molecular switches for any pH: a systematic study of the versatile coordination behaviour of cyclam scorpionands. Chem Eur J 24:1573–1585
Yu D, Du R, Xiao JC, Xu S, Rong C, Liu S (2018) Theoretical study of pKa values for trivalent rare-earth metal cations in aqueous solution. J Phys Chem A 122:700–707
Selwa E, Kenney IM, Beckstein O, Iorga BI (2018) SAMPL6: calculation of macroscopic pKa values from ab initio quantum mechanical free energies. J Computer-Aided Mol Design 32:1203–1216
Pracht P, Wilcken R, Udvarhelyi A, Rodde S, Grimme S (2018) High-accuracy quantum-chemistry-based calculation and blind prediction of macroscopic pKa values in the context of the SAMPL6 challenge. J Computer-Aided Mol Design 32:1139–1149
Lian P, Johnston RC, Parks JM, Smith JC (2018a) Quantum chemical calculation of pKas of environmentally relevant functional groups: carboxylic acids, amines, and thiols in aqueous solution. J Phys Chem A 122:4366–4374
Hu J, Zhao S, Geng W (2018) Accurate pKa computation using matched interface and boundary (MIB) method-based Poisson–Boltzmann solver. Commun Comput Phys 23:520–539
Romero R, Salgado PR, Soto C, Contreras D, Melin V (2018) An experimental validated computational method for pKa determination of substituted 1,2-dihydroxybenzenes. Front Chem 6:208
Li M, Zhang H, Chen B, Wu Y, Guan L (2018) Prediction of pKa values for neutral and basic drugs based on hybrid artificial intelligence methods. Sci Rep 8:3991
Shields G, Seybold P (2014) Computational approaches for the prediction of pKa values. CRC Press, Boca Raton
Reijenga J, van Hoof A, van Loon A, Teunissen B (2013) Development of methods for the determination of pKa values. Anal Chem Insights 8:53–71
Jorgensen WL, Briggs JM, Gao JA (1987) A priori calculations of pKa’s for organic compounds in water. The pKa of ethane. J Am Chem Soc 109:6857
Potter MJ, Gilson MK, McCammon JA (1994) Small molecule pKa prediction with continuum electrostatics calculations. J Am Chem Soc 116:10298
Matsui T, Shigeta Y, Morihashi K (2017) Assessment of methodology and chemical group dependences in the calculation of the pKa for several chemical groups. J Chem Theory Comput 13:4791. https://doi.org/10.1021/acs.jctc.7b00587
Baba T, Matsui T, Kamiya K, Nakano M, Shigeta Y (2014) A density functional study on the pKa of small polyprotic molecules. Int J Quant Chem 114:1128
Rossini E, Netz RR, Knapp EW (2016) Computing pKa values in different solvents by electrostatic transformation. J Chem Theory Comput 12:3360
Han WG, Noodleman L (2010) Quantum cluster size and solvent polarity effects on the geometries and Mssbauer properties of the active site model for ribonucleotide reductase intermediate X: a density functional theory study. Theor Chem Acc 125(3-6):305–317
Haworth NL, Wang Q, Coote ML (2017) Modeling flexible molecules in solution: A pKa case study. J Phys Chem A 121:5217
Sastre S, Casasnovas R, Munoz F, Frau J (2013) Isodesmic reaction for pKa calculations of common organic molecules. Theor Chem Acc 132:1310
Schmidt am Busch M, Knapp EW (2004) Accurate pKa determination for a heterogeneous group of organic molecules. ChemPhysChem 5:1513
Chaudry UA, Popelier PLA (2004) Estimation of pKa using quantum topological molecular similarity descriptors: application to carboxylic acids, anilines and phenols. J Org Chem 69:233
Yu D, Du R, Xiao JC (2016) pKa prediction for acidic phosphorus-containing compounds using multiple linear regression with computational descriptors. J Comput Chem 37:1668
Silva CO, da Silva EC, Nascimento MAC (2000) Ab initio calculations of absolute pKa values in aqueous solution II. Aliphatic alcohols, thiols, and halogenated carboxylic acids. J Phys Chem A 104 (11):2402–2409
Pliego JR (2003) Thermodynamic cycles and the calculation of pKa. Chem Phys Lett 367:145–149
Klamt A, Eckert F, Diedenhofen M, Beck ME (2003) First principles calculations of aqueous pKa values for organic and inorganic acids using COSMO-RS reveal an inconsistency in the slope of the pKa scale. J Phys Chem A 107:9380–9386
Namazian M, Halvani S (2006) Calculations of pKa values of carboxylic acids in aqueous solution using density functional theory. J Thermodyn Chem 38:1495–1502
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phy Chem B 113(18):6378–6396. https://doi.org/10.1021/jp810292n
Lian P, Johnston RC, Parks JM, Smith JC (2018) Quantum chemical calculation of pKa’s of environmentally relevant functional groups: Carboxylic acids, amines and thiols in aqueous solution. J Phy Chem A 122(17):4366–4374. https://doi.org/10.1021/acs.jpca.8b01751
Miertus S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilization of ab-initio molecular potentials for the prevision of solvent effects. Chem Phys 55(1):117–129
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65(2):239–245
Pascual-ahuir JL, Silla E, Tunon I (1994) GEPOL: an improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J Comput Chem 15(10):1127–1138
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum models of solvation. I. General formalism. J Chem Phys 132(11):114110
Foresman JB, Keith TA, Wiberg KB, Snoonian J, Frisch MJ (1996) Solvent effects. 5. Influence of cavity shape, truncation of electrostatics, and electron correlation on ab-initio reaction field calculations. J Phys Chem 100(40):16098–16104
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the c-PCM solvation model. J Comput Chem 24(6):669–681
Klamt A, Schuurmann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc 0(5):799– 805
Klamt A (1995) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99(7):2224–2235
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102(11):1995–2001
Car R, Parrinello M (1985) Unified approach for molecular dynamics and density functional theory. Phys Rev Lett 55:2471
Citra MJ (1999) Estimating the pKa of phenols, carboxylic acids and alcohols from semi-empirical quantum chemical methods. Chemosphere 38 (1):191–206
Czodrowski P, Dramburg I, Sotriffer CA, Klebe G (2006) Development, validation, and application of adapted PEOE charges to estimate pKa values of functional groups in protein-ligand complexes. Proteins: Struct, Funct, Bioinf 65(2):424–437
Hall HK (1957) Correlation of the base strengths of amines1. J Am Chem Soc 79(20):5441–5444
Nogaj B, Dulewicz E, Brycki B, Hrynio A, Barczynski P, Dega-Szafran Z, Szafran M, Koziol P, Katritzky AR (1990) Chlorine-35 nuclear quadrupole resonance and infrared spectroscopic studies of hydrogen bonding in complexes of dichloroacetic acid with nitrogen and oxygen bases: Correlation of spectroscopic properties with proton affinity and aqueous pKa. J Phys Chem 94(4):1279–1285
Charif IE, Mekelleche SM, Villemin D, Mora-Diezc N (2007) Correlation of aqueous pKa values of carbon acids with theoretical descriptors: A DFT study. J Mol Struct: THEOCHEM 818(1-3):1–6
Gasque L, Medina G, Ruiz-Ramirez L, Moreno-Esparza R (1999) Cu-O stretching frequency correlation with phenanthroline pKa values in mixed copper complexes. Inorg Chim Acta 288(1):106–111
Tao L, Han J, Fu-Ming T (2008) Correlations and predictions of carboxylic acid pKa values using intermolecular structure and properties of hydrogen-bonded complexes. J Phys Chem A 112(4):775–782
Psciuk BT, Prémont-Schwarz M, Koeppe B, Keinan S, Xiao D, Nibbering ETJ, Batista VS (2015) Correlating photoacidity to hydrogen-bond structure by using the local O-H stretching probe in hydrogen-bonded complexes of aromatic alcohols. J Phys Chem A 119(20):4800–4812
Sakti AW, Nishimura Y, Nakai H (2018) Rigorous pKa estimation of amine species using density-functional tight-binding-based metadynamics simulations. J Chem Theory Comput 14(1):351–356
Oszczapowicz J (1991) Basicity, H-bonding, Tautomerism and Complex Formation of Imidic Acid Derivatives, Volume 2 edn. Wiley, Ltd., Chichester, Chapter 12, pp 623–688
Huang Y, Liu L, Liu W, Liu S, Liu S (2011) Modeling molecular acidity with electronic properties and Hammett constants for substituted benzoic acids. J Phys Chem A 115:14697–14707
Tomsho JW, Pal A, Hall DG, Benkovic SJ (2012) Ring structure and aromatic substituent effects on the pKa of the benzoxaborole pharmacophore. ACS Med Chem Lett 3:48–52
Kreye WC, Seybold PG (2001) Correlations between quantum chemical indices and the pKa’s of a diverse set of organic phenols. Int J Quant Chem 109(15):3679–3684
Tao Y, Zou W, Cremer D, Kraka E (2017) Characterizing chemical similarity with vibrational spectroscopy: New insights into the substituent effects in monosubstituted benzenes. J Phys Chem A 121(42):8086–8096
Tao Y, Zou W, Cremer D, Kraka E (2017) Correlating the vibrational spectra of structurally related molecules: A spectroscopic measure of similarity. J Comput Chem 39(6):293–306
Konkoli Z, Cremer D (1998) A new way of analyzing vibrational spectra. I. Derivation of adiabatic internal modes. Int J Quantum Chem 67(1):1–9
Wilson EB, Decius JC, Cross PC (1955) Molecular Vibrations. McGraw-Hill, New York
Wilson JrE B (1939) A method of obtaining the expanded secular equation for the vibration frequencies of a molecule. J Chem Phys 7:1047
Kraka E, Freindorf M, Cremer D (2013) Chiral discrimination by vibrational spectroscopy utilizing local modes. Chirality 25(3):185–196
Zhang X, Dai H, Yan H, Zou W, Cremer D (2016b) B-H⋯π interaction: A new type of nonclassical hydrogen bonding. J Am Chem Soc 138(13):4334–4337
Kalescky R, Kraka E, Cremer D (2013) Local vibrational modes of the formic acid dimer—the strength of the double hydrogen bond. Mol Phys 111(9-11):1497–1510
Kalescky R, Zou W, Kraka E, Cremer D (2012) Local vibrational modes of the water dimer—comparison of theory and experiment. Chem Phys Lett 554:243–247
Tao Y, Zou W, Jia J, Li W, Cremer D (2017) Different ways of hydrogen bonding in water—why does warm water freeze faster than cold water? J Chem Theory Comput 13(1):55– 76
Freindorf M, Kraka E, Cremer D (2012) A comprehensive analysis of hydrogen bond interactions based on local vibrational modes. Int J Quantum Chem 112(19):3174–3187
Oliveira V, Kraka E, Cremer D (2016) The intrinsic strength of the halogen bond: Electrostatic and covalent contributions described by coupled cluster theory. Phys Chem Chem Phys 18(48):33031–33046
Oliveira V, Kraka E, Cremer D (2017) Quantitative assessment of halogen bonding utilizing vibrational spectroscopy. Inorg Chem 56(1):488–502
Oliveira V, Cremer D (2017) Transition from metal-ligand bonding to halogen bonding involving a metal as halogen acceptor a study of Cu, Ag, Au, Pt, and Hg complexes. Chem Phys Lett 681:56–63
Setiawan D, Kraka E, Cremer D (2015) Strength of the pnicogen bond in complexes involving group Va Elements N, P, and As. J Phys Chem A 119(9):1642–1656
Setiawan D, Kraka E, Cremer D (2014) Description of pnicogen bonding with the help of vibrational spectroscopy—the missing link between theory and experiment. Chem Phys Lett 614:136–142
Setiawan D, Cremer D (2016) Super-pnicogen bonding in the radical anion of the fluorophosphine dimer. Chem Phys Lett 662:182–187
Oliveira V, Cremer D, Kraka E (2017) The many facets of chalcogen bonding: Described by vibrational spectroscopy. J Phys Chem A 121(36):6845–6862
Sethio D, Oliveira V, Kraka E (2018) Quantitative assessment of tetrel bonding utilizing vibrational spectroscopy. Molecules 23(11):2763
Kalescky R, Kraka E, Cremer D (2014) Description of aromaticity with the help of vibrational spectroscopy: Anthracene and phenanthrene. J Phys Chem A 118(1):223–237
Setiawan D, Kraka E, Cremer D (2016) Quantitative assessment of aromaticity and antiaromaticity utilizing vibrational spectroscopy. J Org Chem 81(20):9669–9686
Li Y, Oliveira V, Tang C, Cremer D, Liu C, Ma J (2017) The peculiar role of the Au3 unit in A u m clusters: σ-aromaticity of the Au5Zn+ ion. Inorg Chem 56(10):5793–5803
Cremer D, Kraka E (2017) Generalization of the tolman electronic parameter: The metal-ligand electronic parameter and the intrinsic strength of the metal-ligand bond. Dalton Transac 46(26):8323–8338
Kalescky R, Kraka E, Cremer D (2014) New approach to Tolman’s electronic parameter based on local vibrational modes. Inorg Chem 53(1):478–495
Setiawan D, Kalescky R, Kraka E, Cremer D (2016) Direct measure of metal–ligand bonding replacing the tolman electronic parameter. Inorg Chem 55(5):2332–2344
Kraka E, Larsson JA, Cremer D (2010). In: Grunenberg J (ed) Computational Spectroscopy: Methods, Experiments and Applications. Wiley, New York
Kalescky R, Kraka E, Cremer D (2013) Identification of the strongest bonds in chemistry. J Phys Chem A 117(36):8981–8995
Kraka E, Setiawan D, Cremer D (2015) Re-evaluation of the bond length-bond strength rule: the stronger bond is not always the shorter bond. J Comp Chem 37(1):130–142
Setiawan D, Kraka E, Cremer D (2015) Hidden bond anomalies: the peculiar case of the fluorinated amine chalcogenides. J Phys Chem A 119(36):9541–9556
Humason A, Zou W, Cremer D (2014) 11,11-dimethyl-1,6-methano[10]annulene-an annulene with an ultralong CC bond or a fluxional molecule?. J Phys Chem A 119(9):1666–1682
Kalescky R, Zou W, Kraka E, Cremer D (2014) Quantitative assessment of the multiplicity of carbon–halogen bonds: carbenium and halonium ions with F, Cl, Br, and I. J Phys Chem A 118(10):1948–1963
Kraka E, Cremer D (2009) Characterization of CF bonds with multiple-bond character: bond lengths, stretching force constants, and bond dissociation energies. ChemPhysChem 10(4):686–698
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Chem Phys 10:6615
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JAJr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian16 Revision A.03. Gaussian Inc., Wallingford
Dennington R, Keith TA, Millam JM (2016) Gaussview Version 5.0.9. Semichem Inc., Shawnee Mission
Dunning TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Phys Chem 90:1007
Woon DE, Dunning TH (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Phys Chem 98:1358
Wilson AK, Woon DE, Peterson KA, Dunning TH (1999) Gaussian basis sets for use in correlated molecular calculations. IX. The atoms gallium through krypton. J Phys Chem 110:7667
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257
Francl MM, Petro WJ, Hehre WJ (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77:3654
Rassolov V, Pople JA, Ratner M, Windus T (1998) 6-31G* basis set for atoms K through Zn. J Chem Phys 109:2257
Ali ST, Karamat S, Kóna J, Fabian WMF (2010) Theoretical prediction of pKa values of seleninic, selenenic, sulfinic, and carboxylic acids by quantum-chemical methods. J Phys Chem A 114 (47):12470–12478
Kraka E, Zou W, Filatov M, Tao Y, Grafenstein J, Izotov D, Gauss J, He Y, Wu A, Konkoli Z (2017) COLOGNE2017. See http://www.smu.edu/catco
Helgaker T, Coriani S, Jorgensen P, Kristensen K, Olsen J, Ruud K (2012) Recent advances in wave function-based methods of molecular-property calculations. Chem Rev 112(1):543–631
D’Alessandro DM, Smit B, Long JR (2010) Carbon dioxide capture: Prospects for new materials. Angew Chem Int Ed 49:6058
MacDowell N, Florin N, Buchard A, Hallett J, Galindo A, Jackson G, Adjiman CS, Williams CK, Shah N, Fennell P (2010) An overview of CO2 capture technologies. Energy Environ Sci 3:1645
Kenarsari SD, Yang D, Jiang G, Zhang S, Wang J, Russell AG, Wei Q, Fan M (2013) Review of recent advances in carbon dioxide separation and capture. RSC Adv 3:22739
Yang X, Rees RJ, Conway W, Puxty G, Yang Q, Winkler DA (2017) Computational modeling and simulation of CO2 capture by aqueous amines. Chem Rev 117:9524–9593
Rochelle GT (2009) Amine scrubbing for CO2 capture. Science 325:1652
Mumford KA, Wu Y, Smith KH, Stevens GW (2015) Review of solvent based carbon-dioxide capture technologies. Front Chem Sci Eng 9:125
Versteeg GF, van Dijck LAJ, van Swaaij WPM (1996) On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions. An overview. Chem Eng Commun 144:113
Puxty G, Rowland R, Allport A, Yang Q, Bown M, Burns R, Maeder M, Attalla M (2009) Carbon dioxide postcombustion capture: A novel screening study of the carbon dioxide absorption performance of 76 amines. Environ Sci Technol 43:6427
Rayer AV, Sumon KZ, Jaffari L, Henni A (2014) Dissociation constants (pKa) of tertiary and cyclic amines: Structural and temperature dependences. J Chem Eng Data 59:3805
Tagiuri A, Mohamedali M, Henni A (2016) Dissociation constant (pKa) and thermodynamic properties of some tertiary and cyclic amines from (298 to 333) K. J Chem Eng Data 61:247
Hamborg ES, Versteeg GF (2009) Dissociation constants and thermodynamic properties of amines and alkanolamines from (293 to 353) K. J Chem Eng Data 54:1318
Manallack DT (2007) The pKa distribution of drugs: Application to drug discovery. Perspectives in Medicinal Chemistry 1:25–38
Charifson PS, Walters WP (2014) Acidic and basic drugs in medicinal chemistry: a perspective. J Med Chem 57:9701–9717
Mitani GM, Steinberg I, Lien EJ, Harrison EC, Elkayam U (1987) The pharmacokinetics of antiarrhythmic agents in pregnancy and lactation. Clin Pharmacokinet 12(4):253–291
Xie X, Steiner SH, Bickel MH (1991) Kinetics of distribution and adipose tissue storage as a function of lipophilicity and chemical structure. II Benzodiazepines. Drug Metab Dispos: The Biological Fate of Chemicals 19 (1):15–9
Avdeef A (2001) Physicochemical profiling (solubility, permeability and charge state). Curr Top Med Chem 1(4):277–351
Kerns E, Di L (2004) Physicochemical profiling: Overview of the screens. Drug Discov. Today Technol. 1 (4):343–348
Acknowledgements
This work was financially supported by National Science Foundation Grants CHE 1464906. We thank SMU for generous supercomputer resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Niraj Verma and Yunwen Tao contributed equally to this work.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, N., Tao, Y., Marcial, B.L. et al. Correlation between molecular acidity (pKa) and vibrational spectroscopy. J Mol Model 25, 48 (2019). https://doi.org/10.1007/s00894-019-3928-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-3928-4