Abstract
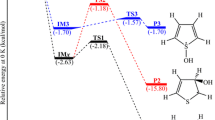
The formation of selenium species in some biological processes involves the generation of ionic and radical intermediates such as RSe●, RSe−, RSeO●, and RSeO−, among others. We performed a theoretical study of the possible mechanisms for the reaction of the two simplest Se radicals—the hydroselenyl (HSe●) and selenenic (HSeO●) radicals, in which the possible products, intermediates, and transition-state structures were investigated. Density functional theory (DFT) was applied at the B3LYP/6–311++G(3df,3pd) level and the Ahlrichs Coulomb fitting basis sets were employed with an effective core potential (ECP) for both Se atoms. The same procedure was used to calculate the electronic density. All calculations were also performed using the M06-2X functional, which describes weaker bonds better than B3LYP does. In the reaction of interest, the so-called CR complex (HSe····SeOH) is formed initially. After passing through the transition state TS1, cis-HSeSeOH is obtained as a product. If a low barrier is then overcome (passing through the transition state TS32), the trans-HSeSeOH species is obtained. The CR complex can also rearrange into the intermediate INT after overcoming the barrier presented by the transition state TS2. Additionally, the decomposition of INT to H2O and 1Se2 is possible through another transition state. This reaction is not included in this study. We also observed a second possible route for the conversion of INT to one of the HSeSeOH species; this route occurs through two pathways (with transition states TS31 and TS32). A comparison of some of the results with those obtained for sulfur analogs along the same pathways is also presented in this work.

Electronic envelopes for HSeO● and HSe● radicals






Similar content being viewed by others
References
Luke BT, McLean AD (1985) A theoretical investigation of atmospheric sulfur chemistry. 1. The HSO/HOS energy separation and the heat of formation of HSO, HOS, and HS2. J Phys Chem 89:4592–4596
Zhang Y, Zhang W, Zhang T, Tian W, Wang W (2012) A computational study on the reaction mechanism of C2H5S with HO2. Comput Theor Chem 994:65–72
Nagy B, Szakács P, Csontos J, Rolik Z, Tasi G, Kállay M (2011) High-accuracy theoretical thermochemistry of atmospherically important sulfur-containing molecules. J Phys Chem A 115:7823–7833
Resende SM, Ornellas FR (2000) Atmospheric reaction between the HS radical and chlorine. Chem Phys Lett 318:340–344
Lemly AD (2004) Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol Environ Saf 59(1):44–56
Lenz M, Lens PNL (2008) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407(12):3620–3633
Staicu LC, Morin-Crini N, Crini G (2017) Desulfurization: critical step towards enhanced selenium removal from industrial effluents. Chemosphere 172:111–119
Domínguez-Álvarez E, Gajdács M, Spengler G, Palop JA, Marć MA, Kieć-Kononowicz K, Amaral L, Molnár J, Jacob C, Handzlik J, Sanmartín C (2016) Identification of selenocompounds with promising properties to reverse cancer multidrug resistance. Bioorg Med Chem Lett 26(12):2821–2824
Flohé L (2009) The labour pains of biochemical selenology: the history of selenoprotein biosynthesis. Biochim Biophys Acta 1790:1389–1403
Allmang C, Wurth L, Krol A (2009) The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim Biophys Acta 1790:1415–1423
Lobanov AV, Hatfield DL, Gladyshev VN (2009) Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta 1790:1424–1428
Castellano S (2009) On the unique function of selenocysteine—insights from the evolution of selenoproteins. Biochim Biophys Acta 1790:1463–1470
Torchinsky YM (1981) Sulfur in proteins. Pergamon, Oxford, pp 1–277
Jacob C, Giles GI, Giles NM, Sies H (2003) Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed 42:4742–4758
Borji S, Vahedpour M, Fazeli S (2016) Mechanistic and energetic study of the atmospheric reaction of hydrosulfinyl and mercapto radicals. Comp Theor Chem 1086:25–35
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Vosko SH, Wilk L, Nusair M (1980) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Can J Phys 58:1200–1211
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Eichkorn K, Treutler O, Ohm OH, Haser M,R, Ahlrichs R (1995) Auxiliary basis sets to approximate Coulomb potentials. Chem Phys Lett 240:283
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor Chem Acc 97:119
Igel-Mann G, Stoll H, Preuss H (1988) Pseudopotentials for main group elements (IIIA through VIIA). Mol Phys 65:1321–1328
Vega-Teijido MA, Zukerman-Schpector J, Ventura ON, Camillo RL, Caracelli I, Guadagnin RC, Braga AL, Silveira CC (2004) Dichloro(cyclohexilidene-1-methylene)(phenyl)Te(IV). Looking for the theoretical treatment. Z Krist 219:652–658
Shulz Lang E, Ledesma G, Abram U, Vega-Teijido MA, Caracelli I, Zukerman-Schpector J (2006) Synthesis, crystal structure and theoretical studies of aryltellurenyltetramethylthiourea (tmtu)iodine complexes: Ph-Te(tmtu)I (1) and β-naphtyl-Te(tmtu)I (2). Z Krist 221:166–172
Caracelli I, Vega-Teijido MA, Zukerman-Schpector J, Cezari MHS, Lopes JGS, Juliano L, Santos PS, Comasseto JV, Cunha RLOR, Tiekink ERT (2012) A tellurium-based cathepsin B inhibitor: molecular structure, modelling, molecular docking and biological evaluation. J Mol Struct 1013:11–18
Gillespie RJ (1970) The electron-pair repulsion model for molecular geometry. J Chem Educ 47(1):18. https://doi.org/10.1021/ed047p18
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J Chem Phys 132:154104
Chéron N, Jacquemin D, Fleurat-Lessard P (2012) A qualitative failure of B3LYP for textbook organic reactions. Phys Chem Chem Phys 14:7170–7175
Todd A, Keith TK (2013) AIMAll (version 13.05.06). Gristmill Software, Overland Park. http://aim.tkgristmill.com/
Acknowledgments
The authors thank the Comisión Sectorial de Investigación Científica-CSIC, UdelaR (CSIC/655) and Programa de Desarrollo de las Ciencias Básicas—PEDECIBA. ONV also thanks the Agencia Nacional de Investigación e Innovación—ANII for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2016
Electronic supplementary material
ESM 1
(DOCX 318 kb)
Rights and permissions
About this article
Cite this article
Vega-Teijido, M.A., Kieninger, M. & Ventura, O.N. Theoretical study of the reactions of the hydroselenyl radical (HSe●) with the selenenic radical (HSeO●). J Mol Model 24, 3 (2018). https://doi.org/10.1007/s00894-017-3535-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3535-1