Abstract
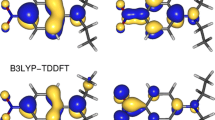
The solvation and the solvatochromic behavior of 5-(dimethylamino)-5′-nitro-2,2′-bithiophene 1, the basis of a π* scale of solvent polarities, was investigated theoretically in toluene, dichloromethane, methanol and formamide with a Monte Carlo and quantum mechanics (QM/MM) iterative approach. The calculated transition energies of the solvatochromic band of 1, obtained as averages of statistically uncorrelated configurations, including the solute and explicit solvent molecules of the first solvation layer, besides showing good agreement with the experimental transitions, reproduced very well the positive solvatochromism of this probe in various solvents.







Similar content being viewed by others
References
Reichardt C (1994) Solvatochromic dyes as solvent polarity indicators. Chem Rev 94(8):2319–2358. doi:10.1021/cr00032a005
Reichardt C, Welton T (2010) Solvent effects on the absorption spectra of organic compounds. Chaper 6 in. Solvents and solvent effects in organic chemistry, 4 edn. Wiley-VCH, Weinheim, pp 359–424. doi:10.1002/9783527632220.ch6
Adegoke OA (2011) Relative predominance of azo and hydrazone tautomers of 4-carboxyl-2,6-dinitrophenylazohydroxynaphthalenes in binary solvent mixtures. Spectrochim Acta A Mol Biomol Spectrosc 83(1):504–510. doi:10.1016/j.saa.2011.08.075
Green AM, Naughton HR, Nealy ZB, Pike RD, Abelt CJ (2013) Carbonyl-twisted 6-acyl-2-dialkylaminonaphthalenes as solvent acidity sensors. J Org Chem 78(5):1784–1789. doi:10.1021/jo301263g
Nandi LG, Facin F, Marini VG, Zimmermann LM, Giusti LA, Rd S, Caramori GF, Machado VG (2012) Nitro-substituted 4-[(phenylmethylene)imino]phenolates: solvatochromism and their use as solvatochromic switches and as probes for the investigation of preferential solvation in solvent mixtures. J Org Chem 77(23):10668–10679. doi:10.1021/jo301890r
Navarro AM, García B, Hoyuelos FJ, Peñacoba IA, Leal JM (2011) Preferential solvation in alkan-1-ol/alkylbenzoate binary mixtures by solvatochromic probes. J Phys Chem B 115(34):10259–10269. doi:10.1021/jp202019x
Papadakis R, Tsolomitis A (2011) Solvatochromism and preferential solvation of 4-pentacyanoferrate 4′-aryl substituted bipyridinium complexes in binary mixtures of hydroxylic and non-hydroxylic solvents. J Solut Chem 40(6):1108–1125. doi:10.1007/s10953-011-9697-z
Salari H, Khodadadi-Moghaddam M, Harifi-Mood AR, Gholami MR (2010) Preferential solvation and behavior of solvatochromic indicators in mixtures of an ionic liquid with some molecular solvents. J Phys Chem B 114(29):9586–9593. doi:10.1021/jp103476a
Sato BM, Martins CT, El Seoud OA (2012) Solvation in aqueous binary mixtures: consequences of the hydrophobic character of the ionic liquids and the solvatochromic probes. New J Chem 36(11):2353–2360. doi:10.1039/C2NJ40506G
Díaz C, Barrio L, Catalán J (2003) Characterization of ternary solvent mixtures: the methanol/ethanol/acetone mixture. Chem Phys Lett 371(5–6):645–654. doi:10.1016/S0009-2614(03)00319-1
Maitra A, Bagchi S (2008) UV-visible spectroscopic study of solvation in ternary solvent mixtures: ketocyanine dye in methanol + acetone + water and methanol + acetone + benzene. J Phys Chem B 112(7):2056–2062. doi:10.1021/jp709819n
Maitra A, Bagchi S (2008) Electronic spectroscopic study of solvation of a ketocyanine dye in ternary solvent mixtures. J Phys Chem B 112(32):9847–9852. doi:10.1021/jp710874e
Ray N, Bagchi S (2005) Use of a solvatochromic probe for study of solvation in ternary solvent mixture. J Phys Chem A 109(1):142–147. doi:10.1021/jp0456741
Ray N, Bagchi S (2004) Fluorimetric study of solvation in ternary solvent mixtures. Ketocyanine dye in ethanol+benzene+water and ethanol+benzene+acetone. J Mol Liq 111(1–3):19–24. doi:10.1016/j.molliq.2003.09.018
Durantini AM, Falcone RD, Silber JJ, Correa NM (2011) A new organized media: glycerol:N, N-dimethylformamide mixtures/AOT/n-heptane reversed micelles. The effect of confinement on preferential solvation. J Phys Chem B 115(19):5894–5902. doi:10.1021/jp1123822
Karukstis KK, Litz JP, Garber MB, Angell LM, Korir GK (2010) A spectral approach to determine location and orientation of azo dyes within surfactant aggregates. Spectrochim Acta A Mol Biomol Spectrosc 75(4):1354–1361. doi:10.1016/j.saa.2009.12.087
Quintana SS, Falcone RD, Silber JJ, Correa NM (2012) Comparison between two anionic reverse micelle interfaces: the role of water–surfactant interactions in interfacial properties. ChemPhysChem 13(1):115–123. doi:10.1002/cphc.201100638
Rezende MC, Oñate R, Núñez G, Domínguez M, Mascayano C (2009) Lipophilic contributions to the solvatochromism of analogous betaines. Dyes Pigments 83(3):391–395. doi:10.1016/j.dyepig.2009.06.011
Vaz Serra V, Andrade SM, Silva EMP, Silva AMS, Neves MGPMS, Costa SMB (2013) Structural effects of the β-vinyl linker in pyridinium porphyrins: spectroscopic studies in organic solvents and AOT reverse micelles. J Phys Chem B 117(48):15023–15032. doi:10.1021/jp4076993
Milosevic P, Hecht S (2005) Design of branched and chiral solvatochromic probes: toward quantifying polarity gradients in dendritic macromolecules. Org Lett 7(22):5023–5026. doi:10.1021/ol0519902
Morgan MT, Carnahan MA, Immoos CE, Ribeiro AA, Finkelstein S, Lee SJ, Grinstaff MW (2003) Dendritic molecular capsules for hydrophobic compounds. J Am Chem Soc 125(50):15485–15489. doi:10.1021/ja0347383
Richter-Egger DL, Landry JC, Tesfai A, Tucker SA (2001) Spectroscopic investigations of polyamido amine starburst dendrimers using the solvatochromic probe phenol blue. J Phys Chem A 105(28):6826–6833. doi:10.1021/jp0100396
Richter-Egger DL, Tesfai A, Tucker SA (2001) Spectroscopic investigations of poly(propyleneimine)dendrimers using the solvatochromic probe phenol blue and comparisons to poly(amidoamine) dendrimers. Anal Chem 73(23):5743–5751. doi:10.1021/ac0155355
Jara F, Domínguez M, Rezende MC (2006) The interaction of solvatochromic pyridiniophenolates with cyclodextrins. Tetrahedron 62(33):7817–7823. doi:10.1016/j.tet.2006.05.053
Mati SS, Sarkar S, Sarkar P, Bhattacharya SC (2012) Explicit spectral response of the geometrical isomers of a bio-active pyrazoline derivative encapsulated in β-cyclodextrin nanocavity: a photophysical and quantum chemical analysis. J Phys Chem A 116(42):10371–10382. doi:10.1021/jp307964z
Naughton HR, Abelt CJ (2013) Local solvent acidities in β-cyclodextrin complexes with PRODAN derivatives. J Phys Chem B 117(12):3323–3327. doi:10.1021/jp400765x
Nicolini J, Venturini CG, Andreaus J, Machado C, Machado VG (2008) Interaction of cyclodextrins with Brooker’s merocyanine in aqueous solution. Spectrosc Lett 42(1):35–41. doi:10.1080/00387010802425142
Sarkar A, Kedia N, Purkayastha P, Bagchi S (2011) Synthesis and spectroscopic investigation of a novel solvatochromic dye. J Lumin 131(8):1731–1738. doi:10.1016/j.jlumin.2011.04.008
Tirapegui C, Jara F, Guerrero J, Rezende MC (2006) Host–guest interactions in cyclodextrin inclusion complexes with solvatochromic dyes. J Phys Org Chem 19(11):786–792. doi:10.1002/poc.1080
Beckford G, Owens E, Henary M, Patonay G (2012) The solvatochromic effects of side chain substitution on the binding interaction of novel tricarbocyanine dyes with human serum albumin. Talanta 92:45–52. doi:10.1016/j.talanta.2012.01.029
Kanski R, Murray CJ (1993) Enzymichromism: determination of the dielectric properties of an enzyme active site. Tetrahedron Lett 34(14):2263–2266. doi:10.1016/S0040-4039(00)77589-7
Khan F, Pickup JC (2013) Near-infrared fluorescence glucose sensing based on glucose/galactose-binding protein coupled to 651-blue oxazine. Biochem Biophys Res Commun 438(3):488–492. doi:10.1016/j.bbrc.2013.07.111
Prifti E, Reymond L, Umebayashi M, Hovius R, Riezman H, Johnsson K (2014) A fluorogenic probe for SNAP-tagged plasma membrane proteins based on the solvatochromic molecule nile red. ACS Chem Biol 9(3):606–612. doi:10.1021/cb400819c
Rezende MC, Dominguez M, Aracena A (2012) The positive halochromism of phenolate dyes in hydroxylic solutions of tetraalkylammonium cations. Spectrochim Acta A Mol Biomol Spectrosc 87:61–66. doi:10.1016/j.saa.2011.11.010
Laus G, Schottenberger H, Wurst K, Schutz J, Ongania K-H, Horvath UEI, Schwarzler A (2003) Solvatochromism, halochromism, and preferential solvation of new dipolar guaiazulenyl 1,4-benzoquinone methides. Org Biomol Chem 1(8):1409–1418. doi:10.1039/B209555F
Rezende MC, Aracena A (2012) In search of the thermo/halochromism of the ET(30) pyridinium-N-phenolate betaine dye. Spectrochim Acta A Mol Biomol Spectrosc 98:18–22. doi:10.1016/j.saa.2012.08.032
Rezende MC, Oñate R, Domínguez M, Millán D (2009) Solvatochromism and halochromism of N-(4-Oxyphenyl) 5-nitro-2-thiophenecarboxaldimine. Spectrosc Lett 42(2):81–86. doi:10.1080/00387010802428617
Zanotto SP, Scremin M, Machado C, Rezende MC (1993) Cationic and anionic halochromism. J Phys Org Chem 6(11):637–641. doi:10.1002/poc.610061108
Lalevée J, Allonas X, Jacques P (2006) Electronic distribution and solvatochromism investigation of a model radical (2,2,6,6-tetramethylpiperidine N-oxyl: tempo) through TD-DFT calculations including PCM solvation. J Mol Struct THEOCHEM 767(1–3):143–147. doi:10.1016/j.theochem.2006.05.054
Cerón-Carrasco JP, Jacquemin D, Laurence C, Planchat A, Reichardt C, Sraïdi K (2014) Determination of a solvent hydrogen-bond acidity scale by means of the solvatochromism of pyridinium-N-phenolate betaine dye 30 and PCM-TD-DFT calculations. J Phys Chem B 118(17):4605–4614. doi:10.1021/jp501534n
Etienne T, Michaux C, Monari A, Assfeld X, Perpète EA (2014) Theoretical computation of betain B30 solvatochromism using a polarizable continuum model. Dyes Pigments 100:24–31. doi:10.1016/j.dyepig.2013.07.017
Silva DL, Murugan NA, Kongsted J, Ågren H, Canuto S (2014) Self-aggregation and optical absorption of stilbazolium merocyanine in chloroform. J Phys Chem B 118(7):1715–1725. doi:10.1021/jp411178h
Silva DL, Barreto RC, Lacerda EG Jr, Coutinho K, Canuto S (2014) One- and two-photon absorption of fluorescein dianion in water: a study using S-QM/MM methodology and ZINDO method. Spectrochim Acta A Mol Biomol Spectrosc 119:63–75. doi:10.1016/j.saa.2013.04.035
Murugan NA (2011) Modeling solvatochromism of a quinolinium betaine dye in water solvent using sequential hybrid QM/MM and semicontinuum approach. J Phys Chem B 115(5):1056–1061. doi:10.1021/jp1049342
Meier H (2005) Conjugated oligomers with terminal donor–acceptor substitution. Angew Chem Int Ed 44(17):2482–2506. doi:10.1002/anie.200461146
Raposo MMM, Sousa AMRC, Kirsch G, Cardoso P, Belsley M, de Matos GE, Fonseca AMC (2006) Synthesis and characterization of dicyanovinyl-substituted thienylpyrroles as new nonlinear optical chromophores. Org Lett 8(17):3681–3684. doi:10.1021/ol061277s
Razus AC, Birzan L, Cristea M, Tecuceanu V, Blanariu L, Enache C (2009) Novel mono- and bis-azo dyes containing the azulen-1-yl moiety: synthesis, characterization, electronic spectra and basicity. Dyes Pigments 80(3):337–342. doi:10.1016/j.dyepig.2008.08.011
Rettig W, Kharlanov V, Effenberger F, Steybe F (2005) Excited state relaxation properties of donor–acceptor-bithiophene and related compounds. Chem Phys Lett 404(4–6):272–278. doi:10.1016/j.cplett.2005.01.096
Domínguez M, Rezende M, Márquez S (2013) Theoretical study of the solvatochromism of a donor-acceptor bithiophene. J Mol Model 19(2):689–696. doi:10.1007/s00894-012-1593-y
Ramirez CB, Carrasco N, Rezende MC (1995) Halochromism and solvatochromism of a [small pi]* probe in binary solvent mixtures. J Chem Soc Faraday Trans 91(21):3839–3842. doi:10.1039/FT9959103839
Effenberger F, Würthner F (1993) 5-dimethylamino-5′-nitro-2, 2′-bithiophene—a new dye with pronounced positive solvatochromism. Angew Chem Int Ed Engl 32(5):719–721. doi:10.1002/anie.199307191
Effenberger F, Wuerthner F, Steybe F (1995) Synthesis and solvatochromic properties of donor-acceptor-substituted oligothiophenes. J Org Chem 60(7):2082–2091. doi:10.1021/jo00112a032
Meng S, Ma J (2008) Solvatochromic shift of donor − acceptor substituted bithiophene in solvents of different polarity: quantum chemical and molecular dynamics simulations. J Phys Chem B 112(14):4313–4322. doi:10.1021/jp710456p
Fukuda R, Ehara M (2014) An efficient computational scheme for electronic excitation spectra of molecules in solution using the symmetry-adapted cluster–configuration interaction method: the accuracy of excitation energies and intuitive charge-transfer indices. J Chem Phys 141(15):154104. doi:10.1063/1.4897561
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford
Domínguez M, Oñate R, Núñez G (2008) MolecV1.0: a program to calculate the number of molecules in a given volume. USACH, Santiago
Jorgensen WL, Madura JD, Swenson CJ (1984) Optimized intermolecular potential functions for liquid hydrocarbons. J Am Chem Soc 106(22):6638–6646. doi:10.1021/ja00334a030
Jorgensen WL (1986) Optimized intermolecular potential functions for liquid alcohols. J Phys Chem 90(7):1276–1284. doi:10.1021/j100398a015
Jorgensen WL, Swenson CJ (1985) Optimized intermolecular potential functions for amides and peptides. Structure and properties of liquid amides. J Am Chem Soc 107(3):569–578. doi:10.1021/ja00289a008
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118(45):11225–11236. doi:10.1021/ja9621760
Coutinho K, Canuto S (2003) DICE:a Monte Carlo program for molecular liquid simulation. University of Sao Paulo, Sao Paulo
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem 11(3):361–373. doi:10.1002/jcc.540110311
Caputo MC, Provasi PF, Benitez L, Georg HC, Canuto S, Coutinho K (2014) Monte Carlo–quantum mechanics study of magnetic properties of hydrogen peroxide in liquid water. J Phys Chem A 118(32):6239–6247. doi:10.1021/jp411303n
Coutinho K, Georg HC, Fonseca TL, Ludwig V, Canuto S (2007) An efficient statistically converged average configuration for solvent effects. Chem Phys Lett 437(1–3):148–152. doi:10.1016/j.cplett.2007.02.012
Georg HC, Coutinho K, Canuto S (2007) Solvent effects on the UV-visible absorption spectrum of benzophenone in water: a combined Monte Carlo quantum mechanics study including solute polarization. J Chem Phys 126(3):034507. doi:10.1063/1.2426346
Reichardt C, Welton T (2010) Empirical parameters of solvent polarity. Chapter 7 in. Solvents and solvent effects in organic chemistry. Wiley-VCH, Weinhiem, pp 425–508. doi:10.1002/9783527632220.ch7
Acknowledgments
We are grateful to Freie Universität Berlin for computational facilities. We are also grateful to Professor Sylvio Canuto for allowing us access to DICE software. M.C.R. acknowledges the Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT project 1140212, and M.D. acknowledges the Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT project 11140497.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1164 kb)
Rights and permissions
About this article
Cite this article
Domínguez, M., Rezende, M.C. A Monte Carlo–quantum mechanics study of a solvatochromic π* probe. J Mol Model 22, 218 (2016). https://doi.org/10.1007/s00894-016-3083-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3083-0