Abstract
Carbohydrates can be used as substrates to synthesize new complex molecules; these molecules contain several chiral centers that can be used in organic synthesis. d-Fucose diphenyl thioacetal reacts differentially with acetone, and this paper describes a study of the mechanism of this reaction using theoretical chemistry methods. The conformer distribution was studied using a Monte Carlo method for the reaction products, and the obtained conformers were validated by calculating the hydrogen spin-spin coupling constants with the DFT/B3LYP/DGDZVP method. Results agreed with the experimental coupling constants with an adequate root mean squared deviation. The free energies and enthalpies of formation of the resulting global minimum conformers were calculated with the same method and with the thermochemical compound method CBS-4 M. This technique, combined with the conformational analysis, allowed comparison of the formation enthalpies of the compounds involved in this reaction, and, with this information, we can postulate the correct reaction pathway.

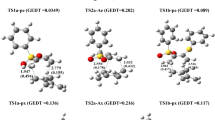
Reaction pathway





Similar content being viewed by others
References
Loraine S, Mendoza-espinoza JA (2010) Las plantas medicinales en la lucha contra el cáncer, relevancia para México. Rev Mex Cienc Farm 41:18–27
Cragg GM, Newman DJ (2005) Plants as a source of anti-cancer agents. J Ethnopharmacol 100:72–79. doi:10.1016/j.jep.2005.05.011
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335. doi:10.1021/np200906s
Mendoza-Espinoza JA, López-Vallejo F, Fragoso-Serrano M et al (2009) Structural reassignment, absolute configuration, and conformation of hypurticin, a highly flexible polyacyloxy-6-heptenyl-5,6-dihydro-2H-pyran-2-one. J Nat Prod 72:700–708. doi:10.1021/np800447k
Bañuelos-Hernandez AE, Mendoza-Espinoza JA (2012) Study of cyclization of diphenylacetals derived from l-rhamnose and l-fucose: a theoretical approach. S Afr J Chem 65:84–90
Bañuelos-Hernández AE, Mendoza-Espinoza JA (2014) Synthesis of the 2,3,4-triacetyl-1,6-dideoxy-l-mannose and tetracetyl-3,6-dideoxy-l-mannitol and the study of the reaction mechanism by molecular modeling. Russ J Gen Chem 84:1450–1457. doi:10.1134/S1070363214070342
Pereda-Miranda R, Fragoso-Serrano M, Cerda-García-Rojas CM (2001) Application of molecular mechanics in the total stereochemical elucidation of spicigerolide, a cytotoxic 6-tetraacetyloxyheptenyl-5,6-dihydro-α-pyrone from Hyptis spicigera. Tetrahedron 57:47–53. doi:10.1016/S0040-4020(00)00987-X
Falomir E, Murga J, Ruiz P et al (2003) Stereoselective synthesis and determination of the cytotoxic properties of spicigerolide and three of its stereoisomers. J Org Chem 68:5672–5676. doi:10.1021/jo034470y
Bañuelos-Hernández AE, Mendoza-Espinoza JA, Pereda-Miranda R, Cerda-García-Rojas CM (2014) Studies of (−)-pironetin binding to α-tubulin: conformation, docking, and molecular dynamics. J Org Chem 79:3752–3764. doi:10.1021/jo500420j
Funabashi M, Arai S, Shinohara M (1999) Novel syntheses of diphenyl and/or trimethylene dithioacetals of mono- and oligosaccharides in 90% trifluoroacetic acid. J Carbohydr Chem 18:333–341. doi:10.1080/07328309908543999
Halgren TA, Nachbar RB (1996) Merck molecular force field. IV. conformational energies and geometries for MMFF94. J Comput Chem 17:587–615. doi:10.1002/(SICI)1096-987X(199604)17:5/6<587::AID-JCC4>3.0.CO;2-Q
Kong J, White CA, Krylov AI et al (2000) Q-Chem 2.0: a high-performanceab initio electronic structure program package. J Comput Chem 21:1532–1548. doi:10.1002/1096-987X(200012)21:16<1532::AID-JCC10>3.0.CO;2-W
Godbout N, Salahub DR, Andzelm J, Wimmer E (1992) Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can J Chem 70:560–571. doi:10.1139/v92-079
Andzelm J, Wimmer E (1992) Density functional Gaussian-type-orbital approach to molecular geometries, vibrations, and reaction energies. J Chem Phys 96:1280. doi:10.1063/1.462165
Frisch MJ, Trucks GW, Schlegel HB et al (2004) GAUSSIAN 03 (Revision B.02). Gaussian, Inc, Wallingford
Smith SG, Goodman JM (2010) Assigning stereochemistry to single diastereoisomers by GIAO NMR calculation: the DP4 probability. J Am Chem Soc 132:12946–12959. doi:10.1021/ja105035r
Zepeda LG, Burgueño-Tapia E, Pérez-Hernández N et al (2013) NMR-based conformational analysis of perezone and analogues. Magn Reson Chem 51:245–250. doi:10.1002/mrc.3940
Pérez-Hernández N, Alvarez-Cisneros C, Cerda-García-Rojas CM et al (2009) Benzylic coupling constants in toluene derivatives by J doubling in the frequency domain and DFT calculations. Magn Reson Chem 47:437–442. doi:10.1002/mrc.2411
Benassi R (2001) A proposed modification of CBS-4M model chemistry for application to molecules of increasing molecular size. Theor Chem Acc Theory Comput Model (Theor Chim Acta) 106:259–263. doi:10.1007/s002140100274
Acknowledgments
Computational resources were provided by the Dirección General de Servicios de Cómputo Académico, Universidad Nacional Autónoma de México. Partial financial support from CONACYT, Mexico (CB-167952), from Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, from Universidad Autónoma de la Ciudad de México, and from Universidad Michoacana de San Nicolás de Hidalgo is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1115 kb)
Rights and permissions
About this article
Cite this article
Bañuelos-Hernandez, A.E., García-Gutiérrez, H.A., Fragoso-Serrano, M. et al. Conformational and reactivity study of dithiophenyl-fucosyl ketals with theoretical chemical methods. J Mol Model 22, 212 (2016). https://doi.org/10.1007/s00894-016-3079-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3079-9