Abstract
The chelating properties of diethanoldithiocarbamate (DEDC) and π-electron flow from the nitrogen atom to the sulfur atom via a plane-delocalized π-orbital system (quasi ring) was studied using a density functional theory method. The molecular structure of DEDC and its complexes with Zn(II), Cd(II), and Hg(II) were also considered. First, the geometries of this ligand and DEDC-Zn(II), DEDC-Cd(II), and DEDC-Hg(II) were optimized, and the formation energies of these complexes were then calculated based on the electronic energy, or sum of electronic energies, with the zero point energy of each species. Formation energies indicated the DEDC-Zn(II) complex as the most stable complex, and DEDC-Cd(II) as the least stable. Structural data showed that the N1–C2 π-bond was localized in the complexes rather than the ligand, and a delocalized π-bond over S7–C2–S8 was also present. The stability of DEDC-Zn(II), DEDC-Cd(II), and DEDC-Hg(II) complexes increased in the presence of the non-specific effects of the solvent (PCM model), and their relative stability did not change. There was π-electron flow or resonance along N1–C2–S7 and along S7–C2–S8 in the ligand. The π-electron flow or resonance along N1–C2–S7 was abolished when the metal interacted with sulfur atoms. Energy belonging to van der Waals interactions and non-covalent delocalization effects between the metal and sulfur atoms of the ligand was calculated for each complex. The results of nucleus-independent chemical shift (NICS) indicated a decreasing trend as Zn(II) < Cd(II) < Hg(II) for the aromaticity of the quasi-rings. Finally, by ignoring van der Waals interactions and non-covalent delocalization effects between the metal and sulfur atoms of the ligand, the relative stability of the complexes was changed as follows:

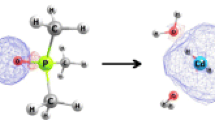
Huge electronic cloud localized on Hg(II) in the Hg(II)-DEDC complex







Similar content being viewed by others
References
Coucouvanis D, Lippard SJ, Zubieta J (1970) The crystal and molecular structure of Bis(ethyl thioanthato)-μ-bis-(ethyl thioxanthato)-μ’-bis(ethylthio)-diiron(III). Inorg Chem 9:2775–2781
Thorn GD, Ludwig RA (1962) The diethanoldithiocarbamates and related compounds. Elsevier, Amsterdam
Hulanicki A (1967) Complexation reactions of diethanoldithiocarbamates. Talanta 14:1371–1392
Ramrakhyani AK, Shukla RS (1980) Synthesis and biological activity of new insecticides. I. Synthesis of N-(−2-mercaptoacetyl-5-aryl-amino, 1,3,4 thiadiazolyl) ureas and N-(S-acetyl-N-aryl dithiocarbamoyl-N3-aryl ureas. J Indian Chem Soc 57:856–857
Kitson T (1985) The diethanoldithiocarbamates—interesting, versatile and neglected. Ed Chem 43–45
Vefforazzi G, Almeida WF, Burin GJ, Jaeger RB, Puga FR, Rahde AF, Reyes FG, Schvartsman S (1995) International safety assessment of pesticides: diethanoldithiocarbamate pesticides, ETU, and PTU—a review and update. Teratog Carcinog Mutagen 15(6):313–337
Weuffen A, Kewitsch A (1967) Connection between chemical constitution and antimicrobial activity. 18. Orientating studies of the tuberculostatic properties of some dithiocarbamic acid esters (dithiourethanes) and dithiocarbamic salts. Arch Expt Veterinaermed 21:1049–1059
Rabbi MF, Finnegan A, Al-Hartli L, Stong S, Roebuck KA (1998) Interleukin-10 enhances tumor necrosis factor-[alpha] activation of HIV-1 transcription in latently infected T cells. Aquired Immune Defic Syndr Hum Retrovirol 19:321–331
Cheng SY, Trombetta L (2004) The induction of amyloid precursor protein and α-synuclein in rat hippocampal astrocytes by diethyldithio carbamate and copper with or without glutathione. Toxicol Lett 146:139–149
Cvek B, Milacic V, Taraba J, Dou QP (2008) Ni(II), Cu(II), and Zn(II) diethyldiethanoldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem 51:6256–6258
Wilton-Ely JDET, Solanki D, Knight ER, Holt KB, Thompson AL, Hogarth G (2008) Multimetallic assemblies using piperazine-based diethanoldithiocarbamate building blocks. Inorg Chem 47:9642–9653
Li Z, Kosov DS (2006) Diethanoldithiocarbamate anchoring in molecular wire junctions: a first principles study. J Phys Chem B 110:9893–9898
Wong WWH, Cookson J, Evans EAL, McInnes EJL, Wolowska J, Maher JP, Bishop P, Beer PD (2005) Heteropolymetallic copper(II)–gold(III) diethanoldithiocarbamate catenanes via magic ring synthesis Beer. Chem Commun 2214–2216
Victoriano LI (2000) The reactivity of metal species towards thiuram sulfides: an alternative route to the syntheses of metal diethanoldithiocarbamates. Coord Chem Rev 196:383–398
Elduque A, Finestra C, Lopez JA, Lahoz FJ, Merchan F, Oro LA, Pinillos MT (1998) Rhodium diethanoldithiocarbamate compounds as metalloligands: a controlled way for the construction of binuclear complexes. Inorg Chem 37:824–829
Coucouvanis D, Swenson D, Stremple P, Baenziger NC (1979) Reaction of [Fe(SC6H5)4]2- with organic trisulfides and implications concerning the biosynthesis of ferredoxins. Synthesis and structure of the [(C6H5)4P]2Fe2S12 complex. J Am Chem Soc 101:3392–3394
Reed AE, Curtiss LA, Wienhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Frisch MJ et al (1998) Gaussian 98 (Revision A.9), Gaussian Inc, Pittsburgh PA
Zahedi M, Bahrami H, Shabazian S, Safari N, Ng SW (2003) An ab initio/hybrid (ONIOM) investigation of biliverdin isomers and metal–biliverdin analogue complexes. J Mol Struct (THEOCHEM) 633:21–33
Lewars EG (2003) Introduction to the theory and applications of molecular and quantum mechanics. Springer, New York
Becke AD (1993) Density‐functional thermochemistry III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Ditchfield W, Hehre WJ, Pople JA (1971) Extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728
Peterson GA, Al-laham MA (1991) A complete basis set model chemistry II. Open‐shell systems and the total energies of the first‐row atoms. J Chem Phys 94:6081–6090
Frisch MJ, Pople JA, Binkley JS (1984) Self-Consistent Molecular Orbital Methods 25: Supplementary Functions for Gaussian Basis Sets. J Chem Phys 80:3265–3269
Beyramabadi SA, Morsali A, Vahidi SH (2012) DFT Characterization of 1-acetylpiperazinyldithiocarbamate ligand and its transition metal complexes. J Struct Chem 53:665–675
De Souza AG, Airoldi C (1987) The thermochemistry of antimony(III) and bismuth(III) diethyldithiocarbamate complexes. Thermochim Acta 130:95–102
Howie RA, Tiekink ERT, Wardell JL, Wardell SMSV (2009) Complementary supramolecular aggregation via O–H · · · O Hydrogen-bonding and Hg · · · S Interactions in Bis[N, N′-di(2-hydroxyethyl)-dithiocarbamato-S, S′]mercury(II): Hg[S2CN(CH2CH2OH)2]2. J Chem Crystallogr 39:293–298
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–241
Schleyer PR, Mareker C, Dransfeld A, Jiao H, Van Eikema Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118(26):6317–6318
Kleinpeter F, Koch A, Shainyan BA (2008) Cyclobutadiene dianion derivatives—planar 4c,6e or three-dimensional 6c,6e aromaticity? J Mol Struct 863:117–122
Alkorta I, Blanco F, Elguero J (2008) Application of Free-Wilson matrices to the analysis of the tautomerism and aromaticity of azapentalenes: a DFT study. Tetrahedron 64:3826–3836
Nielsen MB, Sauer SPA (2008) On the aromaticity of tetrathiafulvalene cations. Chem Phys Lett 453:136–139
Nyulaszi L, Schleyer PR (1999) Hyperconjugative π-aromaticity: how to make cyclopentadiene aromatic. J Am Chem Soc 121:6872–6875
Schleyer PR, Manoharan M, Jiao H, Stahl F (2001) The acenes: is there a relationship between aromatic stabilization and reactivity? Org Lett 3:3643–3646
Acknowledgments
We are grateful to Prof. Seik Weng Ng for making available his software (G98W) and hardware (machine time) facilities. The authors would also like to thank the Research and Graduate Study Councils of Lorestan University for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Highlights
• Complex of transition elements of group 12 with diethanoldithiocarbamate (DEDC) have been considered using theoretical methods.
• Formation energies indicate that stability ordering of complexes is as follows: DEDC − Zn(II) > DEDC − Hg(II) > DEDC − Cd(II)
• The most stabilizing non-bonding interactions and strong forms of van der Waals interaction is in DEDC-Hg(II) due to the huge electron density on Hg(II) in the Hg(II)-DEDC complex.
• The relative stability of complexes due to localized bonding interaction of metal with the sulfur atoms of the ligand (and in the absence of non-bonding interactions and strong forms of van der Waals interactions between metal and sulfur atoms of ligands) is as follows: DEDC − Zn(II) > DEDC – Cd(II) > DEDC – Hg(II)
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.37 mb)
Rights and permissions
About this article
Cite this article
Bahrami, H., Farhadi, S. & Siadatnasab, F. Non-bonding interactions and non-covalent delocalization effects play a critical role in the relative stability of group 12 complexes arising from interaction of diethanoldithiocarbamate with the cations of transition metals Zn(II), Cd(II), and Hg(II): a theoretical study. J Mol Model 22, 155 (2016). https://doi.org/10.1007/s00894-016-3008-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3008-y