Abstract
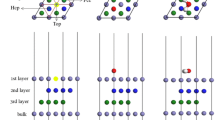
Using density-functional calculations, we studied the interaction between interstitial impurities (N, C) and γ-Fe(111) surfaces doped, or not, with Cr, as well as the effect of Cr doping on the dissolution corrosion resistance of the γ-Fe(111) surface. The elementary processes studied afforded microscopic insights into the formation of a Cr-depleted zone, a phenomenon that leads to local corrosion of the stainless steel surface. The aim of this work was to study, at the atomic scale, the effects of N and C on the segregation behavior of Cr and the synergetic effect between co-doped atoms on the resistance to dissolution corrosion of austenitic stainless steel surfaces. The results showed that interstitial impurities prefer to be trapped at near-surface sites, which can impact the segregation behavior of Cr such that it shifts from the surface to the subsurface. Electrode potential calculations and density of states analysis demonstrated that doping with Cr or inserting interstitial impurities into the solid solution can improve the surface corrosion resistance of an fcc Fe substrate, but detrimental effects on the surface corrosion resistance are induced by interactions between Cr and interstitial impurity atoms in co-doped surfaces. The formation of near-surface Cr carbides and nitrides (speculated to be Cr2N and Cr23C6 due to the results obtained for particular co-doped surfaces) was also noted. The results of our theoretical calculations explain some of the experimental results observed at the atomic scale.







Similar content being viewed by others
References
Tyshchenko AI (2006) Development of stainless austenitic steel alloyed with carbon plus nitrogen. Metallofiz Nov Tekh+ 28:225–233
Bourgin C, Chauveau E, Arnaud A (2006) Effect of a nitrogen addition on mechanical and corrosion properties of 1.4116 martensitic stainless steel. Rev Metall-Paris 103(1):32–36
Christiansen T, Somers MAJ (2005) Low temperature gaseous nitriding and carburising of stainless steel. Surf Eng 21(5–6):445–455. doi:10.1179/174329405x68597
Michal GM, Ernst F, Heuer AH (2006) Carbon paraequilibrium in austenitic stainless steel. Metall Mater Trans A 37A(6):1819–1824. doi:10.1007/s11661-006-0124-9
Stein G, Hucklenbroich I, Feichtinger H (1999) Current and future applications of high nitrogen steels. Mater Sci Forum 318–320:151–160. doi:10.4028/www.scientific.net/MSF.318-320.151
Mudali UK, Khatak HS, Raj B, Uhlemann M (2004) Surface alloying of nitrogen to improve corrosion resistance of steels and stainless steels. Mater Manuf Process 19(1):61–73. doi:10.1081/Amp-120027501
Yang K, Ren YB (2010) Nickel-free austenitic stainless steels for medical applications. Sci Technol Adv Mat 11:014105. doi 10.1088/1468-6996/11/1/014105
Rokanopoulou A, Papadimitriou GD (2011) Production of high nitrogen surfaces on 2205 duplex stainless steel substrate using the PTA technique. Mater Sci Tech-Lond 27(9):1391–1398. doi:10.1179/026708310x12712410311974
Ha H, Jang H, Kwon H, Kim S (2009) Effects of nitrogen on the passivity of Fe-20Cr alloy. Corros Sci 51(1):48–53. doi:10.1016/j.corsci.2008.10.017
Fukunaga T, Kaneko K, Kawano R, Ueda K, Yamada K, Nakada N, Kikuchi M, Barnard JS, Midgley PA (2014) Formation of intergranular M23C6 in sensitized type-347 stainless steel. Isij Int 54(1):148–152. doi:10.2355/isijinternational.54.148
Martinavicius A, Abrasonis G, Scheinost AC, Danoix R, Danoix F, Stinville JC, Talut G, Templier C, Liedke O, Gemming S, Moller W (2012) Nitrogen interstitial diffusion induced decomposition in AISI 304L austenitic stainless steel. Acta Mater 60(10):4065–4076. doi:10.1016/j.actamat.2012.04.014
Ha H, Kwon H (2007) Effects of Cr2N on the pitting corrosion of high nitrogen stainless steels. Electrochim Acta 52(5):2175–2180. doi:10.1016/j.electacta.2006.08.034
Zheng LG, Hu XQ, Kang XH, Li DZ (2013) Precipitation behavior of M23c6 and its effects on ductility and toughness of a noveL Cr-Mn-N austenitic heat resistant steel. Acta Metall Sin 49(9):1081–1088. doi:10.3724/Sp.J.1037.2013.00198
Samal MK, Abhishek A (2011) A new model for the prediction of chromium depletion near grain boundaries and corresponding sensitization in austenitic stainless steels. P I Mech Eng C-J Mec 225(C4):809–815. doi:10.1243/09544062jmes2327
Kaneko K, Fukunaga T, Yamada K, Nakada N, Kikuchi M, Saghi Z, Barnard JS, Midgley PA (2011) Formation of M23C6-type precipitates and chromium-depleted zones in austenite stainless steel. Scripta Mater 65(6):509–512. doi:10.1016/j.scriptamat.2011.06.010
Yu XF, Chen SH, Wang L (2009) Effect of solution treatment conditions on the sensitization of austenitic stainless steel. J Serb Chem Soc 74(11):1293–1302. doi:10.2298/Jsc0911293y
Bennekom PRLA (1995) The involvement of alloyed nitrogen in the corrosion of stainless steels. J South Afric Inst Mining Metall 95(7):337–346
Fu N, Tang XH, Li DY, Parent L, Tian H (2015) In situ investigation of local corrosion at interphase boundary under an electrochemical-atomic force microscope. J Solid State Electr 19(2):337–344. doi:10.1007/s10008-014-2601-1
Tokunaga T, Ohtani H, Agren J (2011) Evaluation of sensitization and self-healing in austenitic stainless steels based on simulations of Cr-depleted zones. Isij Int 51(6):965–968
Yu XF, Chen SH, Wang L (2009) Simulation of recrystallization in cold worked stainless steel and its effect on chromium depletion by cellular automaton. Comput Mater Sci 46(1):66–72. doi:10.1016/j.commatsci.2009.02.008
Riedinger R, Dreyssé H (1985) Electronic structure of interstitial impurities near surfaces. Phys Rev B 31(6):3398–3404. doi:10.1103/PhysRevB.31.3398
Jiang D, Carter E (2003) Carbon dissolution and diffusion in ferrite and austenite from first principles. Phys Rev B 67 (21). doi:10.1103/PhysRevB.67.214103
Yu XF, Chen SH (2009) A simulation of Cr depletion in austenitic stainless steel with cellular automaton. Comput Mater Sci 45(4):899–904. doi:10.1016/j.commatsci.2008.12.012
Turpin T, Dulcy J, Gantois M (2005) Carbon diffusion and phase transformations during gas carburizing of high-alloyed stainless steels: experimental study and theoretical modeling. Metall Mater Trans A 36A(10):2751–2760. doi:10.1007/s11661-005-0271-4
Gavriljuk VG, Shanina BD, Berns H (2008) Ab initio development of a high-strength corrosion-resistant austenitic steel. Acta Mater 56(18):5071–5082. doi:10.1016/j.actamat.2008.06.021
Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MIJ, Refson K, Payne MC (2005) First principles methods using CASTEP. Z Krist 220(5/6/2005):567–570. doi:10.1524/zkri.220.5.567.65075
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):A1133–A1138
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136(3B):B864–B871
John P, Perdew KB, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:18
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41(11):7892–7895. doi:10.1103/PhysRevB.41.7892
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192
Pfrommer BGMC, Louie SG, Cohen ML (1997) Relaxation of crystals with the quasi-Newton method. J Comp Phys 131:233–240
Häglund J, Fernández Guillermet A, Grimvall G, Körling M (1993) Theory of bonding in transition-metal carbides and nitrides. Phys Rev B 48(16):11685–11691
Basinski ZS, Hume-Rothery W, Sutton AL (1955) The lattice expansion of iron. Proc R Soc Lond Ser A 229(1179):459–467. doi:10.1098/rspa.1955.0102, citeulike-article-id:10762171
Lee S-J, Lee Y-K, Soon A (2012) The austenite/ɛ-martensite interface: a first-principles investigation of the fcc Fe/hcp Fe(0001) system. Appl Surf Sci 258(24):9977–9981. doi:10.1016/j.apsusc.2012.06.059
Jiang Q, Lu HM, Zhao M (2004) Modelling of surface energies of elemental crystals. J Phys Condens Matter 16(4):521–530. doi:10.1088/0953-8984/16/4/001
Suzuki STK, Inoue H, Isshiki M, Waseda Y (1996) Effect of the surface segregation of chromium on oxidation of high-purity Fe-Cr alloys at room temperature. Appl Surf Sci 103:495–502
Tyson WR, Miller WA (1977) Surface free energies of solid metals: estimation from liquid surface tension measurements. Surf Sci 62(1):267–276. doi:10.1016/0039-6028(77)90442-3
Kiejna A, Wachowicz E (2008) Segregation of Cr impurities at bcc iron surfaces: first-principles calculations. Phys Rev B 78:113403. doi:10.1103/PhysRevB.78.113403
Ponomareva AV, Isaev EI, Skorodumova NV, Vekilov YuKh, Abrikosov IA (2007) Surface segregation energy in bcc Fe-rich Fe-Cr alloys. Phys Rev B. doi: 10.1103/PhysRevB.75.245406
Idczak R, Idczak K, Konieczny R (2014) Oxidation and surface segregation of chromium in Fe-Cr alloys studied by Mossbauer and X-ray photoelectron spectroscopy. J Nucl Mater 452(1–3):141–146. doi:10.1016/j.jnucmat.2014.05.003
Zhao W, Wang JD, Lu FB, Chen DR (2009) First principles study of H2O molecule adsorption on Fe(100), Fe(110) and Fe(111) surfaces. Acta Phys Sin-Ch Ed 58(5):3352–3358
Greeley J, Nørskov JK (2007) Electrochemical dissolution of surface alloys in acids: thermodynamic trends from first-principles calculations. Electrochim Acta 52(19):5829–5836. doi:10.1016/j.electacta.2007.02.082
Ma YG, Balbuena PB (2008) Surface properties and dissolution trends of Pt3M alloys in the presence of adsorbates. J Phys Chem C 112(37):14520–14528. doi:10.1021/Jp8046888
Hörmann NG, Jäckle M, Gossenberger F, Roman T, Forster-Tonigold K, Naderian M, Sakong S, Groß A (2015) Some challenges in the first-principles modeling of structures and processes in electrochemical energy storage and transfer. J Power Sources 275:531–538. doi:10.1016/j.jpowsour.2014.10.198
Gossenberger F, Roman T, Groß A (2015) Equilibrium coverage of halides on metal electrodes. Surf Sci 631:17–22. doi:10.1016/j.susc.2014.01.021
Ernst F, Cao Y, Michal GM, Heuer AH (2007) Carbide precipitation in austenitic stainless steel carburized at low temperature. Acta Mater 55(6):1895–1906. doi:10.1016/j.actamat.2006.09.049
Michal G, Ernst F, Kahn H, Cao Y, Oba F, Agarwal N, Heuer A (2006) Carbon supersaturation due to paraequilibrium carburization: stainless steels with greatly improved mechanical properties. Acta Mater 54(6):1597–1606. doi:10.1016/j.actamat.2005.11.029
Zhang ZR, Zhao ZY, Li CZ, Jiang ZH, Li HB (2013) Effects of aging precipitates on the mechanical and corrosion resistance properties of 18Cr-18Mn-2Mo-0.96N super high nitrogen austenitic stainless steel. Appl Mech Mat 395–396:284–288. doi: 10.4028/www.scientific.net/AMM.395-396.284
Acknowledgments
This work was financially supported by the National Science Foundation of China (nos. 51371123, 51304145), the Specialized Research Foundation of the Doctoral Program for Institution of Higher Education (nos. 2013140211003, 20131402120004), the Natural Science Foundation of Shanxi Province (nos. 2014011002–1, 2014021018–1, 2013011010–1, 2013021013–1), and the Opening Fund of the State Key Laboratory of Nonlinear Mechanics.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, C., Zhang, C., Liu, X. et al. DFT study of the effects of interstitial impurities on the resistance of Cr-doped γ-Fe(111) surface dissolution corrosion. J Mol Model 21, 206 (2015). https://doi.org/10.1007/s00894-015-2755-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2755-5