Abstract
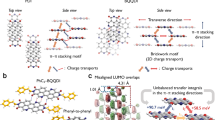
The nitrogen doping and phenyl substitution effects on the geometries, molecular stacking character, electronic, and charge transport properties of tetrabenzoheptacene (TTBH) have been investigated by means of density functional theory (DFT) calculation and incoherent charge hopping model. Our results indicate that the nitrogen doping (TTH) at the 6,8,15,17 positions improves its stability in air and the ability of electron injection and in the meantime slightly changes the molecular stacking due to the C-H···N interaction. For both TTBH and TTH, large hole transport mobility (μ h ) and electron transport mobility (μ e ), which are on the same order of magnitude, are given rise by their dense displaced π-stacking in crystal. Comparatively, the phenyl substitution (Ph-TTBH) at the 6,8,15,17 positions adopts a non-planar conformation, adverse to close packing and therefore leads to smaller electron/hole transport mobility (μ) than those of TTBH and TTH. The calculations suggest TTBH and TTH are promising candidates for excellent ambipolar OFET materials.

In comparison with parent tetrabenzoheptacenes compound, the C-H···N hydrogen bonds interaction caused by nitrogen doping gives rise to different relative in-plane displacement of the dimer with shortest centroid distance in crystal structure. Such distinguish leads to the significantly different frontier molecular orbital interaction of monomers in the dimer, which explains the different nature of the charge transfer







Similar content being viewed by others
References
Tang CW, Vanslyke SA (1987) Appl Phys Lett 5:913–915
Slyke SAV, Chen CH, Tang CW (1996) Appl Phys Lett 69:2160–2162
Mas-Torrent M, Rovira C (2008) Chem Soc Rev 37:827–838
Shirota Y, Kageyama H (2007) Chem Rev 107:953–1010
Garnier F (1998) Chem Phys 227:253–262
Klauk H (2010) Chem Soc Rev 39:2643–2666
Lin Y, Li YF, Zhan XW (2012) Chem Soc Rev 41:4245–4272
Hains AW, Liang ZQ, Woodhouse MA, Gregg BA (2010) Chem Rev 110:6689–6735
O’Neill M, Kelly SM (2011) Adv Mater 23:566–584
Anthony JE (2008) Angew Chem Int Ed Engl 47:452–483
Anthony JE (2006) Chem Rev 106:5028–5048
Bendikov M, Wudl F (2004) Chem Rev 104:4891–4945
Kivelson S, Chapman OL (1983) Phys Rev B 28:7236–7243
Kertesz M, Lee YS, Stewart JJP (1989) Int J Quantum Chem 35:305–313
Biermann D, Schmidt W (1980) J Am Chem Soc 102:3163–3173
Mateo-Alonso A (2014) Chem Soc Rev 43:6311–6324
More S, Bhosale R, Choudhary S, Mateo-Alonso A (2012) Org Lett 14:4170–4173
Choudhary S, Gozalvez C, Higelin A, Krossing I, Melle-Franco M, Mateo-Alonso A (2014) Chem Eur J 20:1525–1528
More S, Bhosale R, Mateo-Alonso A (2014) Chem Eur J 20:10626–10631
Kulisic N, More S, Mateo-Alonso A (2011) Chem Commun 47:514–516
Clar E (1964) Polycyclic hydrocarbons. Springer, London, p 200
Duong HM, Bendikov M, Steiger D, Zhang QC, Sonmez G, Yamada J, Wudl F (2003) Org Lett 5:4433–4436
Mateo-Alonso A, Kulisic N, Valenti G, Marcaccio M, Paolucci F, Prato M (2010) Chem Asian J 5:482–485
Tauber MJ, Kelley RF, Giaimo JM, Rybtchinski B, Wasielewski MR (2006) J Am Chem Soc 128:1782–1783
Coropceanu V, Cornil J, da Silva Filho DA, Olivier Y, Silbey R, Bredas J-L (2007) Chem Rev 107:926–952
Wu QX, Geng Y, Liao Y, Tang XD, Yang GC, Su ZM (2012) Theor Chem Acc 131:1121–1129
Zhao CB, Wang WL, Yin SW, Ma Y (2013) New J Chem 37:2925–2934
Zhao CB, Guo YL, Guan L, Ge HG, Yin SW, Wang WL (2014) Synth Met 188:146–155
Marcus RA (1993) Rev Mod Phys 65:599–610
Hush NS (1958) J Chem Phys 28:962–972
Bredas JL, Calbert JP, da Silva Filho DA (2002) J Cornil Proc Natl Acad Sci USA 99:5804–5809
Cornil J, Lemaur V, Calbert J-P, Bredas J-L (2002) Adv Mater 14:726–729
Zhang YX, Cai X, Bian YZ, Li XY, Jiang JZ (2008) J Phys Chem C 112:5148–5159
Huang J-D, Wen S-H, Deng W-Q, Han K-L (2011) J Phys Chem B 115:2140–2147
Peng Q, Yi YP, Shuai ZG, Shao JS (2007) J Am Chem Soc 129:9333–9339
Nan GJ, Yang XD, Wang LJ, Shuai ZG, Zhao Y (2009) Phys Rev B 79:115203–115211
Yin SW, Li LL, Yang YM, Reimers JR (2012) J Phys Chem C 116:14826–14836
Norton JE, Bredas J-L (2008) J Am Chem Soc 130:12377–12384
Brovchenko IV (1997) Chem Phys Lett 278:355–359
McMahon DP, Troisi A (2010) J Phys Chem Lett 1:941–946
Malagoli M, Bredas JL (2000) Chem Phys Lett 327:13–17
Lemaur V, da Silva Filho DA, Coropceanu V, Lehmann M, Geerts Y, Piris J, Debije MG, van de Craats AM, Senthilkumar K, Siebbeles LDA, Warman JM, Bredas J-L, Cornil J (2004) J Am Chem Soc 126:3271–3279
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, CossiM SG, RegaN PGA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2010) Gaussian 09, Revision C.01. Gaussian, Inc, Wallingford
Kuo M-Y, Chen H-Y, Chao I (2007) Chem Eur J 13:4750–4758
Geng Y, Wang JP, Wu SX, Li HB, Yu F, Yang GC, Gao HZ, Shuai ZG (2011) J Mater Chem 21:134–143
Yang XD, Wang LJ, Wang CL, Long W, Shuai ZG (2008) Chem Mater 20:3205–3211
Yang XD, Li QK, Shuai ZG (2007) Nanotechnology 18:424029–424034
Schein LB (1979) Phys Rev B 20:1631–1639
Li CH, Huang CH, Kuo MY (2011) Phys Chem Chem Phys 13:11148–11155
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839–845
Song YB, Di CA, Yang XD, Li SP, Xu W, Liu YQ, Yang LM, Shuai ZG, Zhang DQ, Zhu DB (2006) J Am Chem Soc 128:15940–15941
Huang JS, Kertesz M (2004) Chem Phys Lett 390:110–115
Gao H-Z (2010) Synth Met 160:2104–2108
Winkler M, Houk KN (2007) J Am Chem Soc 129:1805–1815
He ZK, Mao RX, Liu DQ, Miao Q (2012) Org Lett 14:4190–4193
Chen HY, Chao I (2006) Chem Phys Chem 7:2003–2007
Tang M, Oh JH, Reichardt AD, Bao Z (2009) J Am Chem Soc 131:3733–3740
Chen X-K, Guo J-F, Zou L-Y, Ren A-M, Fan J-X (2011) J Phys Chem C 115:21416–21428
Yasuda T, Goto T, Fujita K, Tsutsui T (2004) Appl Phys Lett 85:2098–2100
Michaelson HB (1977) J Appl Phys 48:4729–4733
Acknowledgments
This work is supported by the National Nature Science Foundation of China (21173139, 21173138, 21473108), the Fundamental Research Funds for the Central Universities (No: GK201101004, GK201303004), and the Shaanxi Innovative Team of Key Science and Technology (2013KCT-17).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 25648 kb)
Rights and permissions
About this article
Cite this article
Guan, L., Wang, W., Shao, R. et al. Molecular stacking character and charge transport properties of tetrabenzoheptacenes derivatives: the effects of nitrogen doping and phenyl substitution. J Mol Model 21, 126 (2015). https://doi.org/10.1007/s00894-015-2677-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2677-2