Abstract
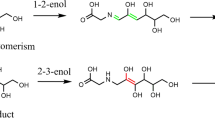
The mechanism of Maillard reaction has been investigated by means of density functional theory calculations in the gaseous phase and aqueous solution. The Maillard reaction is a cascade of consecutive and parallel reaction. In the present model system study, glucose and glycine were taken as the initial reactants. On the basis of previous experimental results, the mechanisms of Maillard reaction have been proposed, and the possibility for the formation of different compounds have been evaluated through calculating the relative energy changes for different steps of reaction under different pH conditions. Our calculations reveal that the TS3 in Amadori rearrangement reaction is the rate-determining step of Maillard reaction with the activation barriers of about 66.7 and 68.8 kcal mol-1 in the gaseous phase and aqueous solution, respectively. The calculation results are in good agreement with previous studies and could provide insights into the reaction mechanism of Maillard reaction, since experimental evaluation of the role of intermediates in the Maillard reaction is quite complicated.




















Similar content being viewed by others
References
Maillard LC (1912) Acad Sci Ser 154:66–68
Davidek J, Velisek J, Pokorny J (1990) Development in food science: chemical changes during food processing. Elsevier, Amsterdam
Eskin NAE (2000) Biochemistry of foods. Academic, San Diego
Macrane R, Robinson RK, Saadler MJ (1993) Encyclopedia of food science, food technology and nutrition. Academic, London
Ledl F, Schleicher E (1990) Angew Chem Int Ed 29:565–594
Warshel A (1991) Computer modelling of chemical reactions in enzymes and solutions. Wiley, New York
Prasad BR, Plotnikov NV, Warshel A (2013) J Phys Chem B 117:153–163
Kamerlin SCL, Sharma PK, Prasad RB, Warshel A (2013) Q Rev Biophys 46:1–132
Kamerlin SCL, Warshel A (2011) Comput Mol Sci 1:30–45
Fors S (1983) ACS Symp Ser 215:185–286
Friedman M (1996) J Agric Food Chem 44:631–653
Finot PA, Aeschbacher HU, Hurrell RF, Liardon R (eds) (1990) The Maillard reaction in food processing, human nutrition and physiology. Birkäuser, Basel
Morales FJ, Jimenez-Perez S (2001) Food Chem 72:119–125
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S (2002) Nature 419:449–450
Mottram DS, Wedzicha BL, Dodson AT (2002) Nature 419:448–449
Kaanane A, Labuza TP (1989) The Maillard reaction in foods. In: Baynes JW, Monnier VM (eds) The Maillard reaction in aging, diabetes and nutrition. Alan Rliss, New York, pp 301–327
Hodge JE (1953) J Agric Food Chem 1:928–943
Van Boekel MAJS (1996) J Food Sci 61:477–489
Yaylayan VA (1997) Trends Food Sci Technol 8:13–18
Martins SIFS, Van Boekel MAJS, Jongen WMF (2000) Czech J Food Sci 18:281–282
Ghiron AF, Quack B, Mahinney TP, Feather MS (1988) J Agric Food Chem 36:673–676
Tressl R, Nittka C, Kersten E (1995) J Agric Food Chem 43:1163–1169
Yaylayan VA, Huyghues-Despointes A (1996) J Agric Food Chem 44:672–681
Van Boekel MAJS, Brands C (1998) Heating of sugar-casein solutions: isomerization and Maillard reactions. In: Brien OJ, Nursten HE, Crabbe MJC, Ames JM (eds) The Maillard reaction in foods and medicine. Royal Society of Chemistry, Cambridge, pp 154–158
Van Boekel MAJS (2001) Nahrung/Food 45:150–159
Rizzi GP (2003) J Agric Food Chem 51:1728–1731
Du QQ, Song FR, Liu ZQ, Liu SY (2010) Acta Chim Sin 13:1331–1336
Jokic A, Zimpel Z, Huang PM, Mezey PG (2001) SAR QSAR Environ Res 12:297–307
Jalbout AF, Shipar MAH, Navarro JL (2007) Food Chem 103:919–926
Patel S, Rabone J, Russell S, Tissen J, Klaffke W (2001) J Chem Inf Comput Sci 41:926–933
Ericksson C ed (1981) Maillard reaction in food: chemical, physiological and technological aspects. In: Progress in food and nutrition science 5, Pergamon, Oxford
Waller GR, Feather MS (eds) (1983) The Maillard reaction in foods and nutrition, ACS Symp Ser 215, Washington DC
Fujimaki M, Namiki M, Kato H (eds) (1986) Amino-carbonyl reactions in foods and biological systems. Developments in food science 13. Elsevier, Amsterdam
O′Brien J, Nursten HE, Crabbe MJC, Ames JM (eds) (1998) The Maillard reaction in foods and medicine. Royal Society of Chemistry, Cambridge
Beck AD (2003) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Miehlich B, Savin A, Stoll H, Preuss H (1989) Chem Phys Lett 157:200–206
Wang GC, Pan YM, Gao QY, Zhao XZ (1996) Chin J Chem Phys 9:406–411
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comp Chem 24:669–681
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1 Gaussian Inc, Wallingford
Zhao Y, Truhlar DG (2008) Theor Chem Accounts 120:215–241
Ho CT (1996) The Maillard reaction: consequences for the chemical and life sciences. Ikan R (ed) Wiley, New York, p 27
Macrane R, Robinson RK, Saadler MJ (eds) (1993) Encyclopedia of food science, food technology and nutrition, vol 1. Academic, London
Jalbout AF, Roy AK, Shipar MAH, Ahmed MS (2008) Int J Quantum Chem 108:589–597
Shipar MAH (2006) Food Chem 98:395–402
Koenig PH, Ghosh N, Hoffmann M, Elstner M, Tajkhorshid E, Frauenheim T, Cui Q (2006) J Phys Chem A 110:548–563
Cao ZX, Mo YR, Thiel W (2007) Angew Chem Int Ed 46:6811–6815
Galesa K, Bren U, Kranjc A, Mavri J (2008) J Agric Food Chem 56:8720–8727
Bren U, Zupan M, Guengerich FP, Mavri J (2006) J Org Chem 71:4078–4084
Acknowledgments
The author acknowledges financial support from the National Science Foundation of China (21203166, 21302167, 21473157), the Natural Science Foundation of Zhejiang Province (Y4100620), and the Food Science and Engineering the Most Important Discipline of Zhejiang Province (JYTsp2014111).
Compliance with ethical standards
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ren, GR., Zhao, LJ., Sun, Q. et al. Explore the reaction mechanism of the Maillard reaction: a density functional theory study. J Mol Model 21, 132 (2015). https://doi.org/10.1007/s00894-015-2674-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2674-5