Abstract
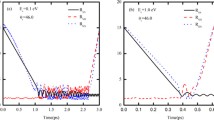
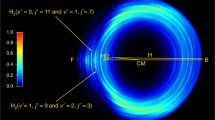
Quasiclassical trajectory calculations based on a fully global ab initio potential energy surface of the rotational angular momentum polarisation of the product CO in the title reaction are reported. The alignment and orientation of the rotational angular momentum of the CO fragment in the scattering frame were found to be sensitive to the initial collision energy chosen. Differences in the angular momentum polarization at different collision energies were traced to differences in the microscopic reaction mechanism. The results of this study suggest that the title reaction is mainly dominated by an abstraction reaction mechanism (involving the short-lived and metastable intermediate complex COH) at low collision energies; however, at relatively high energies, an insertion reaction mechanism (involving the long-lived and stable intermediate complex HCO) plays a role.

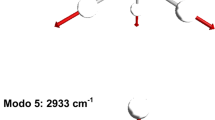
Schematic of the abstraction mechanism, which leads to the orientation of the angular momentum of the product CO along the positive y-axis. The H atom is red, the C atom is yellow, and the O atom is green. Crosses and circles represent directions into and out of the scattering plane






Similar content being viewed by others
References
Lee SH, Liu K (2000) In: Campargue R (ed) Advances in molecular beam research and applications. Springer, Berlin
Casavecchia P (2000) Rep Prog Phys 63:355
Smith IWM, Herbst E, Chang Q (2004) Mon Not R Astron Soc 350:323
Carty D, Goddard A, Kohler SPK, Sims IR, Smith IWM (2006) J Phys Chem A 110:3101
Graff MM (1989) Astrophys J 339:239
Zanchet A, Bussery-Honvault B, Honvault P (2006) J Phys Chem A 110:12017
Zanchet A, Halvick P, Rayez JC, Bussery-Honvault B, Honvault P (2007) J Chem Phys 126:184308
Zanchet A, Halvick P, Bussery-Honvault B, Honvault P (2008) J Chem Phys 128:204301
Bussery-Honvault B, Dayou F, Zanchet A (2008) J Chem Phys 129:234302
Lin SY, Guo H, Honvault P (2008) Chem Phys Lett 453:140
Gray SK, Balint-Kurti GG (1998) J Chem Phys 108:950
Balint-Kurti GG, Gonzalez AI, Goldfield EM, Gray SK (1998) Faraday Discuss 110:169
Hankel M, Balint-Kurti GG, Gray SK (2003) Int J Quantum Chem 92:205
Bulut N, Zanchet A, Honvault P, Bussery-Honvault B, Bañares L (2009) J Chem Phys 130:194303
Song P, Zhu YH, Liu JY, Ma FC (2010) J Theor Comput Chem 9:935
Ding YJ, Shi Y (2011) Comput Theor Chem 963:306
Alexander AJ, Aoiz FJ, Bañares L, Brouard M, Simons JP (2000) Phys Chem Chem Phys 2:571
Zhang L, Chen MD, Wang ML, Han KL (2000) J Chem Phys 112:3710
Chu TS, Zhang Y, Han HK (2006) Int Rev Phys Chem 25:201
Li XH, Wang MS, Pino I, Yang CL, Ma LZ (2009) Phys Chem Chem Phys 11:10438
Han B, Zong FJ, Wang CL, Ma WY, Zhou JH (2010) Chem Phys 374:94
Huang YR (2014) J Mol Model 20:2151
Orr-Ewing AJ, Zare RN (1994) Annu Rev Phys Chem 45:315
De Miranda MP, Clary DC (1997) J Chem Phys 106:4509
Aoiz FJ, Brouard M, Enriquez PA (1996) J Chem Phys 105:4964
Han KL, He GZ, Lou NQ (1996) J Chem Phys 105:8699
Alexander AJ, Aoiz FJ, Bañares L, Brouard M, Short J, Simons JP (1997) J Phys Chem A 101:7544
Han KL, Zhang L, Xu DL, He JZ, Lou NQ (2001) J Phys Chem A 105:2956
Ma JJ, Chen MD, Cong SL, Han KL (2006) Chem Phys 327:529
Ju LP, Han KL, Zhang JZH (2009) J Comput Chem 30:305
Brouard M, Lambert HM, Rayner SP, Simons JP (1996) Mol Phys 89:403
Wang ML, Han KL, He GZ (1998) J Chem Phys 109:5446
De Miranda MP, Aoiz FJ, Banares L, Sáez-Rábanos V (1999) J Chem Phys 111:5368
Aoiz FJ, Bañares L, Herrero VJ (1998) J Chem Soc Faraday Trans 94:2483 (and references therein)
Shafer-Ray NE, Orr-Ewing AJ, Zare RN (1995) J Phys Chem 99:7591
Aoiz FJ, Bañares L, Herrero VJ (2006) J Phys Chem A 110:12546
Boggio-Pasqua M, Voronin AI, Halvick P, Rayez JC (2000) Phys Chem Chem Phys 2:1693
Halvick P, Boggio-Pasqua M, Bonnet L, Voronin AI, Rayez JC (2002) Phys Chem Chem Phys 4:2560
Dayou F, Spielfiedel A (2003) J Chem Phys 119:4237
Acknowledgments
This work is supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant no. 11404154) and the Scientific Research Foundation of the Education Department of Liaoning Province, China (grant no. L2013149)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Y. Effect of collision energy on the reaction mechanism of C(3P) + OH(X 2 Π) → CO(X 1 Σ +) + H(2S). J Mol Model 21, 103 (2015). https://doi.org/10.1007/s00894-015-2634-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2634-0