Abstract
We report the results of a density functional theory investigation of the bonding of nucleobases mediated by silver and gold atoms in the gas phase. Our calculations use the Becke exchange and Perdew–Wang correlation functional (BPW91) combined with the Stuttgart effective core potentials to represent the valence electrons of gold, silver, and platinum, and the all-electron DGTZVP basis set for C, H, N, and O. This combination was chosen based on tests on the metal atoms and tautomers of adenine, cytosine, and guanine. To establish a benchmark to understand the metal-mediated bonding, we calculated the binding energy of each of the base pairs in their canonical forms. Our calculations show rather strong bonds between the Watson–Crick base pairs when compared with typical values for N–H–N and N–H–O hydrogen bonds. The neutral metal atoms tend to bond near the nitrogen atoms. The effect of the metal atoms on the bonding of nucleobases differs depending on whether or not the metal atoms bond to one of the hydrogen-bonding sites. When the silver or gold atoms bond to a non-hydrogen-bonding site, the effect is a slight enhancement of the cytosine–guanine bonding, but there is almost no effect on the adenine–thymine pairing. The metal atoms can block one of the hydrogen-bonding sites, thus preventing the normal cytosine–guanine and adenine–thymine pairings. We also find that both silver and gold can bond to consecutive guanines in a similar fashion to platinum, albeit with a significantly lower binding energy.

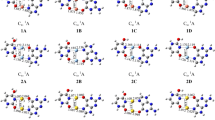
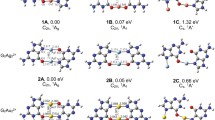
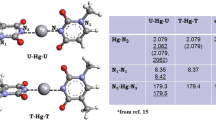
We report the results of a density functional theory investigation of the bonding of nucleobases mediated by silver and gold atoms in the gas phase. Our calculations use the Becke exchange and Perdew–Wang correlation functional (BPW91) combined with the Stuttgart effective core potentials to represent the valence electrons of gold, silver, and platinum, and the all-electron DGTZVP basis set for C, H, N, and O. This combination was chosen based on tests on the metal atoms and on tautomers of adenine, cytosine, and guanine. To establish a benchmark to understand the metal-mediated bonding, we calculated the binding energy of each of the base pairs in their canonical forms. Our calculations show rather strong bonds between the Watson–Crick base pairs when compared with typical values for N–H–N and N–H–O hydrogen bonds. The neutral metal atoms tend to bond near the nitrogen atoms. The effect of the metal atoms on the bonding of nucleobases differs depending on whether or not the metal atoms bond to one of the hydrogen-bonding sites. When the silver or gold atoms bond to a non-hydrogen-bonding site, the effect is a slight enhancement of the cytosine–guanine bonding, but there is almost no effect on the adenine–thymine pairing. The metal atoms can block one of the hydrogen-bonding sites, thus preventing the normal cytosine–guanine and adenine–thymine pairings. We also find that both silver and gold can bond to consecutive guanines in a similar fashion to platinum, albeit with a significantly lower binding energy. Stable gas-phase structures are shown in the figure: a guanine–Ag–guanine, b guanine–Au–guanine, and c guanine–Pt–guanine complexes













Similar content being viewed by others
References
Lippert B (1999) Impact of cisplatin on the recent development of Pt coordination chemistry: a case study. Coord Chem Rev 182:263–295
Lippert B (2000) Multiplicity of metal ion binding patterns to nucleobases. Coord Chem Rev 200–202:487–516
Kryacko ES, Remacle F (2005) Complexes of DNA bases and Watson–Crick base pairs with small neutral gold clusters. J Phys Chem B 109:22746–22757
Lu G, Wei F, Jiang H et al (2009) DFT study on the intermolecular interactions between Au n (n = 2–4) and thymine. J Mol Struc THEOCHEM 915:98–104
Martinez A (2009) Do anionic gold clusters modify conventional hydrogen bonds? The interaction of anionic Au n (n = 2–4) with adenine–uracil base pair. J Phys Chem A 113:1134–1140
Shukla MK, Dubey M, Zakar E et al (2009) DFT investigation of the interaction of gold nanoclusters with nucleic acid base guanine and Watson–Crick guanine–cytosine base pair. J Phys Chem C 113:3960–3966
Sponer J, Burda JV, Sabat M et al (1998) Interaction between the guanine–cytosine Watson–Crick DNA base pair and hydrated group IIa (Mg2+, Ca2+, Sr2+, Ba2+) and group IIb (Zn2+, Cd2+, Hg2+) metal cations. J Phys Chem A 102:5951–5857
Burda JV, Sponer J, Leszczynski J et al (1997) Interaction of DNA base pairs with various metal cations (Mg2+, Ca2+, Sr2+, Ba2+, Cu+, Ag+, Au+, Zn2+, Cd2+, and Hg2+): nonempirical ab initio calculations on structures, energies, and nonadditivity of the interaction. J Phys Chem B 101:9670–9677
Schreiber M, González L (2007) Structure and bonding of Ag(I)–DNA base complexes and Ag(I)–adenine–cytosine mispairs: an ab initio study. J Comp Chem 28:2299–2308
Yang B, Wau RR, Polfer NC et al (2013) IRMPD action spectroscopy of alkali metal cations-sytosine complexes: effects of alkali metal cation size on gas phase conformations. J Am Soc Mass Spec 24:1523–1533
Berdakin M, Steinmetz V, Maitre P et al (2014) Gas phase structure of metal mediated (cytosine)2Ag+ mimics the hemiprotonated (cytosine)2H+ dimer in i-motif folding. J Phys Chem A 118:3804–3809
Vrkic AK, Taverner T, James PF et al (2004) Gas phase ion chemistry of charged silver(I) adenine ions via multistage mass spectrometry experiments and DFT calculations. Dalton Trans 2004:197–208
Soto-Verdugo V, Metiu H, Gwinn E (2010) The properties of small Ag clusters bound to DNA bases. J Chem Phys 132:195102
Preuss M, Schmidt WG, Bechstedt F (2005) Coulombic amino group–metal bonding: adsorption of adenine on Cu(110). Phys Rev Lett 94:236102–236105
Rauls E, Blankenburg S, Schmidt WG (2008) DFT calculations of adenine adsorption on coin metal (110) surfaces. Surf Sci 602:2170–2174
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098–3100
Perdew JP, Wang Y (1992) Accurate and simple analytic representation of the electron-gas correlation energy. Phys Rev B 45:13244–13249
Frisch MJ et al (2004) Gaussian 03. Gaussian, Inc., Wallingford
Andrae D, Haussermann U, Dolg M et al (1990) Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor Chim Acta 77:123–141
Godbout N, Salahub DR, Andzelm J, Wimmer E (1992) Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can J Chem 70(2):560–57
Sosa C, Andzelm J, Elkin BC, Wimmer E, Dobbs KD, Dixon DA (1992) J Phys Chem 96(16):6630–6636
Acioli PH, Burkland S, Srinivas S (2012) Exploration of the potential energy surface of the seven atom silver cluster and the carbon monoxide ligand. Eur Phys J D 66:215–222
Singh RM, Ortiz JV, Mishra MK (2010) Tautomeric forms of adenine: vertical ionization energies and Dyson orbitals. Int J Quant Chem 110:1901–1915
Hanus M, Ryjacek F, Kabelac M et al (2004) Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment, and in aqueous solution. Part 3. Adenine. J Phys Chem B 108:2087–2097
Trygubenko SA, Bogdan TV, Rueda M et al (2002) Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Part 1. Cytosine. Phys Chem Phys 4:4192–4203
Kobayashi R (1998) A CCSD (T) study of the relative stabilities of cytosine tautomers. J Phys Chem A 102:10813–10817
Fogarasi G (2002) Relative stabilities of three low-energy tautomers of cytosine: a coupled cluster electron correlation study. J Phys Chem A106:1381–1390
Marian CM (2007) The guanine tautomer puzzle: quantum chemical investigation of ground and excited states. J Phys Chem A 111(8):1545–1553
Hanus M, Ryjacek KM, Kubar T et al (2003) Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in microhydrated environment and in aqueous solution. Guanine: surprising stabilization of rare tautomers in aqueous solution. J Am Chem Soc 125:7678–7688
Rejnek J, Hanus M, Kabelac M et al (2005) Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Part 4. Uracil and thymine. Phys Chem Phys 7:2006–2017
Civcir PU (2000) A theoretical study of tautomerism of cytosine, thymine, uracil and their 1-methyl analogues in the gas and aqueous phases using AM1 and PM3. J Mol Struc THEOCHEM 532:157–169
Wu Z, Ono A, Kainosho M et al (2001) H…N hydrogen bond lengths in double stranded DNA from internucleotide dipolar couplings. J Biomol NMR 19:361–365
Kimura-Suda H, Petrovykh DY, Tarlov MJ (2003) Base dependent competitive adsorption of single-stranded DNA on gold. J Am Chem Soc 125:9014–9015
Estarella C, Frontera A, Quiñonero AI et al (2009) Energetic vs synergetic stability: a theoretical study. J Phys Chem A 113:3266–3273
Carloni P, Sprik M, Andreoni W (2000) Key steps of the cisplatin–DNA interaction: density functional theory-based molecular dynamics simulations. J Phys Chem B 104(4):823–835
Senthilnathan D, Vaideeswaran S, Venuvanalingam P et al (2011) Antitumor activity of bent metallocenes: electronic structure analysis using DFT computations. J Mol Mod 17:465–475
Chval Z, Kabelac M, Burda JV (2013) Mechanism of the cis-[Pt(1R,2R‑DACH)(H2O) 2] 2+ intrastrand binding to the double-stranded (pGpG)·(CpC) dinucleotide in aqueous solution: a computational DFT study. Inorg Chem 52:5801–5813
Chiavarino B, Crestoni ME, Fornarini S et al (2013) Interaction of cisplatin with adenine and guanine: a combined IRMPD, MS/MS and theoretical study. J Am Chem Soc 135:1445–1455
Acknowledgments
We acknowledge the financial support provided by an Extramural Associate Research Development Award (EARDA) Type G11 grant (5G11HD049644-03) from the National Institutes of Health and administered by Northeastern Illinois University, Chicago, IL, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection QUITEL 2013
Rights and permissions
About this article
Cite this article
Acioli, P.H., Srinivas, S. Silver- and gold-mediated nucleobase bonding. J Mol Model 20, 2391 (2014). https://doi.org/10.1007/s00894-014-2391-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2391-5