Abstract
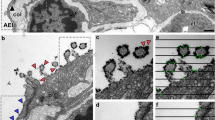
The glycocalyx (GCX) covers the luminal surface of blood vessels and regulates vascular permeability. As GCX degradation predicts various types of vasculopathy, confirming the presence of this structure is useful for diagnosis. Since the GCX layer is very fragile, careful fixation is necessary to preserve its structure. We explored appropriate and feasible methodologies for visualizing the GCX layer using lung tissue specimens excised from anesthetized mice. Each specimen was degassed and immersed in Alcian blue (ALB) fixative solution, and then observed using electron microscopy. Specimens from septic mice were prepared as negative GCX controls. Using these immersion-fixed specimens, the GCX layer was successfully observed using both transmission and scanning electron microscopy; these observations were similar to those obtained using the conventional method of lanthanum perfusion fixation. Spherical aggregates of GCX were observed in the septic mouse specimens, and the GCX density was lower in the septic specimens than in the non-septic specimens. Of note, the presently reported methodology reduced the specimen preparation time from 6 to 2 days. We, therefore, concluded that our novel method could be applied to human lung specimens and could potentially contribute to the further elucidation of vasculopathies.






Similar content being viewed by others
References
Ushiyama A, Kataoka H, Iijima T (2016) Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care 4:59
Kataoka H, Ushiyama A, Akimoto Y, Matsubara S, Kawakami H, Iijima T (2017) Structural behavior of the endothelial glycocalyx is associated with pathophysiologic status in septic mice: an integrated approach to analyzing the behavior and function of the glycocalyx using both electron and fluorescence intravital microscopy. Anesth Analg 125:874–883
Becker BF, Chappell D, Brugger D, Annecke T, Jacob M (2010) Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 87:300–310
Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF (2014) Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesthesiol 31:474–481
Shinohara A, Ushiyama A, Iijima T (2021) Time-dependent dynamics required for the degradation and restoration of the vascular endothelial glycocalyx layer in lipopolysaccharide-treated septic mice. Front Cardiovasc Med 8:730298
Fernandez-Sarmiento J, Molina CF, Salazar-Pelaez LM, Flórez S, Alarcón-Forero LC, Sarta M, Hernández-Sarmiento R, Villar JC (2022) Biomarkers of glycocalyx injury and endothelial activation are associated with clinical outcomes in patients with sepsis: a systematic review and meta-analysis. J Intensive Care Med 38:95–105
Astapenko D, Tomasova A, Ticha A, Hyspler R, Chua HS, Manzoor M, Skulec R, Lehmann C, Hahn RG, Malbrain ML, Cerny V (2022) Endothelial glycocalyx damage in patients with severe COVID-19 on mechanical ventilation—a prospective observational pilot study. Clin Hemorheol Microcirc 81:205–219
Okada H, Takemura G, Suzuki K, Oda K, Takada C, Hotta Y, Miyazaki N, Tsujimoto A, Muraki I, Ando Y, Zaikokuji R, Matsumoto A, Kitagaki H, Tamaoki Y, Usui T, Doi T, Yoshida T, Yoshida S, Ushikoshi H, Toyoda I, Ogura S (2017) Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit Care 21:261
Mukai S, Takaki T, Nagumo T, Sano M, Kang D, Takimoto M, Honda K (2021) Three-dimensional electron microscopy for endothelial glycocalyx observation using Alcian blue with silver enhancement. Med Mol Morphol 54:95–107
Thomas RC, Bath MF, Stover CM, Lambert DG, Thompson JP (2014) Exploring LPS-induced sepsis in rats and mice as a model to study potential protective effects of the nociceptin/orphanin FQ system. Peptides 61:56–60
Braber S, Verheijden KA, Henricks PA, Kraneveld AD, Folkerts G (2010) A comparison of fixation methods on lung morphology in a murine model of emphysema. Am J Physiol Lung Cell Mol Physiol 299:L843-851
Karasutani K, Baskoro H, Sato T, Arano N, Suzuki Y, Mitsui A, Shimada N, Kodama Y, Seyama K, Fukuchi Y, Takahashi K (2019) Lung fixation under constant pressure for evaluation of emphysema in mice. J Vis Exp 151:e58197
Inagawa R, Okada H, Takemura G, Suzuki K, Takada C, Yano H, Ando Y, Usui T, Hotta Y, Miyazaki N, Tsujimoto A, Zaikokuji R, Matsumoto A, Kawaguchi T, Doi T, Yoshida T, Yoshida S, Kumada K, Ushikoshi H, Toyoda I, Ogura S (2018) Ultrastructural alteration of pulmonary capillary endothelial glycocalyx during endotoxemia. Chest 154:317–325
Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, Zaikokuji R, Hotta Y, Miyazaki N, Yano H, Muraki I, Kuroda A, Fukuda H, Kawasaki Y, Okamoto H, Kawaguchi T, Watanabe T, Doi T, Yoshida T, Ushikoshi H, Yoshida S, Ogura S (2018) Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep 8:17523
Tachi M, Okada H, Matsuhashi N, Takemura G, Suzuki K, Fukuda H, Niwa A, Tanaka T, Mori H, Hara A, Yoshida K, Ogura S, Tomita H (2019) Human colorectal cancer infrastructure constructed by the glycocalyx. J Clin Med 8:1270
Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, Rubashkin MG, Magbanua MJ, Thorn KS, Davidson MW, Rugo HS, Park JW, Hammer DA, Giannone G, Bertozzi CR, Weaver VM (2014) The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511:319–325
Nishida R, Suzuki D, Akimoto Y, Matsubara S, Hayakawa J, Ushiyama A, Sasa K, Miyamoto Y, Iijima T, Kamijo R (2023) Exploring the pathophysiological mechanism of interstitial edema focusing on the role of macrophages and their interaction with the glycocalyx. J Oral Biosci 65:111–118
Yamaoka-Tojo M (2020) Vascular endothelial glycocalyx damage in COVID-19. Int J Mol Sci 21:9712
Cortese K, Holland G, Möller L, Gagliani MC, Barisione E, Ball L, Pelosi P, Grillo F, Mastracci L, Fiocca R, Laue M (2022) Ultrastructural examination of lung “cryobiopsies” from a series of fatal COVID-19 cases hardly revealed infected cells. Virchows Arch 480:967–977
Suzuki K, Okada H, Takemura G, Takada C, Tomita H, Yano H, Muraki I, Zaikokuji R, Kuroda A, Fukuda H, Nishio A, Takashima S, Suzuki A, Miyazaki N, Fukuta T, Yamada N, Watanabe T, Doi T, Yoshida T, Kumada K, Ushikoshi H, Yoshida S, Ogura S (2020) Recombinant thrombomodulin protects against LPS-induced acute respiratory distress syndrome via preservation of pulmonary endothelial glycocalyx. Br J Pharmacol 177:4021–4033
Yini S, Heng Z, Xin A, Xiaochun M (2015) Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol Scand 59:160–169
Yang Y, Haeger SM, Suflita MA, Zhang F, Dailey KL, Colbert JF, Ford JA, Picon MA, Stearman RS, Lin L, Liu X, Han X, Linhardt RJ, Schmidt EP (2017) Fibroblast growth factor signaling mediates pulmonary endothelial glycocalyx reconstitution. Am J Respir Cell Mol Biol 56:727–737
Tang Y, Wang X, Li Z, He Z, Yang X, Cheng X, Peng Y, Xue Q, Bai Y, Zhang R, Zhao K, Liang F, Xiao X, Andersson U, Wang H, Billiar TR, Lu B (2021) Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity 54:454–467
Iba T, Levy JH, Aihara K, Kadota K, Tanaka H, Sato K, Nagaoka I (2020) Newly developed recombinant antithrombin protects the endothelial glycocalyx in an endotoxin-induced rat model of sepsis. Int J Mol Sci 22:176
Li X, Zhu J, Liu K, Hu Y, Huang K, Pan S (2020) Heparin ameliorates cerebral edema and improves outcomes following status epilepticus by protecting endothelial glycocalyx in mice. Exp Neurol 330:113320
Wang L, Huang X, Kong G, Xu H, Li J, Hao D, Wang T, Han S, Han C, Sun Y, Liu X, Wang X (2016) Ulinastatin attenuates pulmonary endothelial glycocalyx damage and inhibits endothelial heparanase activity in LPS-induced ARDS. Biochem Biophys Res Commun 478:669–675
Chevalier L, Selim J, Genty D, Baste JM, Piton N, Boukhalfa I, Hamzaoui M, Pareige P, Richard V (2017) Electron microscopy approach for the visualization of the epithelial and endothelial glycocalyx. Morphologie 101:55–63
Van den Berg BM, Vink H, Spaan JA (2003) The endothelial glycocalyx protects against myocardial edema. Circ Res 92:592–594
Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoeckelhuber M, Conzen P, Becker BF (2009) The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res 104:1313–1317
Yang CY, Huynh T, Johnson M, Gong H (2014) Endothelial glycocalyx layer in the aqueous outflow pathway of bovine and human eyes. Exp Eye Res 128:27–33
Acknowledgements
We thank Mr. Oniki and Ms. Nagai for their support with the specimen preparation at Showa University. We thank KAC Co. for preparing the paraffin-embedded blocks and slides. We thank Ms. Myrna Harrod-Taniguti for the English proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Conventional perfusion fixation with lanthanum nitrate
SEM
A solution consisting of 2.5% glutaraldehyde (Nisshin-EM Co. Tokyo, Japan), 2% sucrose, and 2% lanthanum nitrate (Sigma-Aldrich Japan, Tokyo, Japan) was used as the perfusion fixative.
The mice were deeply anesthetized using an intramuscular injection of a cocktail of ketamine and xylazine. A perfusion pump was used to perform the injection at a steady rate of 1 mL/min for about 15–20 min. The lungs were then harvested and diced into 5 mm cubes.
Each cube was immersed in the perfusion solution for one day of fixation at 4 ℃. The specimens were then dehydrated using a graded ethanol series. Samples were freeze-cracked at the critical point, osmium-coated, and observed using SEM at an acceleration voltage of 15 kV, a spot size of 40, and the high vacuum mode [8, 27,28,29,30].
TEM
Perfusion-fixed lungs were diced into 1-mm cubes and immersed in 2.5% glutaraldehyde, 2% sucrose, and 2% lanthanum nitrate fixing solution at 4 ℃ for one day. The specimens were then washed with 30 mM HEPES (DOJINDO LABORATORIES, Japan) and fixed in 2% osmium tetroxide at 4 ℃ for 2 h.
Specimens were dehydrated in a graded ethanol series and embedded in epoxy resin. After ultra-thin sectioning, the specimens were stained with 2% uranyl acetate and 2.7% lead citrate and were observed using TEM at an acceleration voltage of 80 kV [2, 9, 17].
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wakatsuki, M., Takaki, T., Ushiyama, A. et al. Fast-track preparation of lung specimens for electron microscope observations of the pulmonary endothelial glycocalyx. Med Mol Morphol 56, 239–249 (2023). https://doi.org/10.1007/s00795-023-00360-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-023-00360-1