Abstract
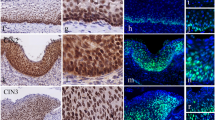
Accumulated evidence has shown that endocan, which was originally called endothelial cell-specific molecule-1, is an attractive prognostic factor in a variety of cancers. However, the relevance of endocan expression in human malignancies remains to be clarified. In the present study, the expression of endocan in cervical squamous neoplasia of the uterus, including low- and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively), as well as in invasive squamous cell carcinoma was examined by immunohistochemistry. Endocan was not sufficiently expressed in the normal cervical epithelium. Endocan expression was present in LSIL cases but was limited to basal and parabasal areas of the cells. HSIL cases exhibited strong expression of endocan with widely distributed expression toward the epithelial surface. In contrast, further strong expression of endocan was not observed in patients with invasive carcinoma. This study is the first study showing increased expression of endocan in precancerous dysplastic lesions and malignancy of the cervix. The data suggest that a high expression level of endocan potentially contributes to the development of cervical squamous neoplasia of the uterus.


Similar content being viewed by others
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB (1996) ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem 271:20458–20464
Zhang SM, Zuo L, Zhou Q, Gui SY, Shi R, Wu Q, Wei W, Wang Y (2012) Expression and distribution of endocan in human tissues. Biotech Histochem 87:172–178
Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M (2006) Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta 1765:25–37
Öztop N, Özer PK, Demir S, Beyaz S, Tiryaki TO, Özkan G, Aydogan M, Bugra MZZ, Çolakoglu B, Büyüköztürkn S, Nalçacı M, Yavuz AS, Gelincik A (2021) Impaired endothelial function irrespective of systemic inflammation or atherosclerosis in mastocytosis. Ann Allergy Asthma Immunol 127:76–82
Rocha SF, Schiller M, Jing D, Li H, Butz S, Vestweber D, Biljes D, Drexler HCA, Nieminen-Kelhä M, Vajkoczy P, Adams S, Benedito R, Adams RH (2014) sm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ Res 115:581–590
Lu J, Liu Q, Zhu L, Liu Y, Zhu X, Peng S, Chen M, Li P (2022) Endothelial cell-specific molecule 1 drives cervical cancer progression. Cell Death Dis 13:1043
Yang J, Yang Q, Yu S, Zhang X (2005) Endocan: a new marker for cancer and a target for cancer therapy. Biomed Rep 3:279–283
Cui Y, Guo W, Li Y, Shi J, Ma S, Guan F (2021) Pan-cancer analysis identifies ESM1 as a novel oncogene for esophageal cancer. Esophagus 18:326–338
Kano K, Sakamaki K, Oue N, Kimura Y, Hashimoto I, Hara K, Maezawa Y, Aoyama T, Fujikawa H, Hiroshima Y, Yamada T, Tamagawa H, Yamamoto N, Ogata T, Cho H, Ito H, Shiozawa M, Yukawa N, Yoshikawa T, Morinaga S, Rino Y, Yasui W, Masuda M, Miyagi Y, Oshima T (2020) ESM-1 gene expression on outcomes in stage II/III gastric cancer patients who received adjuvant S-1 chemotherapy. Vivo 34:461–467
Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, de Vries NJ, Heideveld M, Nieuwkoop PD (1989) Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340:140–144
Means A, Gudas LJ (1995) The roles of retinoids in vertebrate development. Annu Rev Biochem 64:210–233
Petkovich M, Brand NJ, Krust A, Chambon P (1987) A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 330:444–450
Osanai M, Sawada N, Lee GH (2010) Oncogenic and cell survival properties of the retinoic acid metabolizing enzyme, CYP26A1. Oncogene 29:1135–1144
Osanai M (2017) Cellular retinoic acid bioavailability in various pathologies and its therapeutic implication. Pathol Int 67:281–289
Osanai M, Takasawa A, Takasawa K, Kyuno D, Ono Y, Magara K (2023) Retinoic acid metabolism in cancer: potential feasibility of retinoic acid metabolism blocking therapy. Med Mol Morphol 56:1–10
Van heusden J, Wouters W, Ramaekers FC, Krekels MD, Dillen L, Borgers M, Smets G, (1998) The antiproliferative activity of all-trans-retinoic acid catabolites and isomers is differentially modulated by liarozole-fumarate in MCF-7 human breast cancer cells. Br J Cancer 77:1229–1235
Sonneveld E, van den Brink CE, van der Leede BM, Schulkes RK, Petkovich M, van der Burg B, van der Saag PT (1998) Human retinoic acid (RA) 4-hydroxylase (CYP26) is highly specific for all-trans-RA and can be induced through RA receptors in human breast and colon carcinoma cells. Cell Growth Diff 9:629–637
Iaassen I, Brakenhoff RH, Smeets SJ, Snow GB, Braakhuis BJ (2001) Metabolism and growth inhibition of four retinoids in head and neck squamous normal and malignant cells. Br J Cancer 85:630–635
Ozpolat B, Mehta K, Tari AM, Tari AM, Lopez-Berestein G (2002) All-trans-retinoic acid-induced expression and regulation of retinoic acid 4-hydroxylase (CYP26) in human promyelocytic leukemia. Am J Hematol 70:39–47
Shelton DN, Sandoval IT, Eisinger A, Chidester S, Ratnayake A, Ireland CM, Jones DA (2006) Up-regulation of CYP26A1 in adenomatous polyposis coli-deficient vertebrates via a WNT-dependent mechanism: implications for intestinal cell differentiation and colon tumor development. Cancer Res 66:7571–7577
WHO Classification of Tumours Editorial Board 2020 Female genital tumours. International Agency for Research on Cancer (WHO classification of tumours Lyon series, 5th ed.; vol.4)
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458
Straight SW, Hinkle PM, Jewers RJ, McCance DJ (1993) The E5 oncoprotein of human papilloma virus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J Virol 67:4521–4532
Nagy A, Munkacsy G, Gyorffy B (2021) Pancancer survival analysis of cancer hallmark genes. Sci Rep 11:6047
Acknowledgements
Certain parts of this study were included in the Japanese language PhD thesis of the authors MS and AI at Sapporo Medical University School of Medicine.
Funding
No specific funding was received. This study was supported in part by education and research funds of Sapporo Medical University School of Medicine.
Author information
Authors and Affiliations
Contributions
MS, AI, and MO substantially contributed to the conception and design of this study. MS, AI, AT, KT, DK, and KM performed histological examination of the cervical neoplasia and performed immunohistochemistry. MS, AI, and MO confirmed the authenticity of all of the raw data obtained. MS and AI were major contributors to data analysis and interpretation of the data. MS and MO contributed to manuscript drafting and critical revisions on the intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that we have no conflicts of interests.
Ethics approval and consent to participate
The present study was reviewed and approved by the Institutional Ethics Committee (approval no. 4-1-44) and Institutional Review Board (study no. 312-230) of Sapporo Medical University. The Ethics Committee waived the requirement to obtain written informed consent from the patients for the use of human tissues owing to the retrospective nature of the study. The research was conducted in accordance with the Declaration of Helsinki. The researchers involved in this study had no access to information that could identify individual participants during or after data collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sato, M., Inoue, A., Takasawa, A. et al. Elevated expression of endocan in the development of cervical squamous neoplasia of the uterus. Med Mol Morphol 56, 187–193 (2023). https://doi.org/10.1007/s00795-023-00353-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-023-00353-0