Abstract
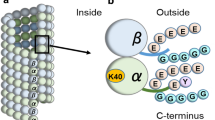
Acetylation of α-tubulin is a well-studied posttranscriptional modification, which is mostly catalyzed by α-tubulin N-acetyltransferase (ATAT1). ATAT1 possibly affects various cellular functions related with microtubules, such as intracellular transport, cell motility, cilia formation, and neuronal signaling. Here, we analyzed the subcellular localization of immunolabeled ATAT1 in human fibroblast KD cells through the cell cycle using confocal laser scanning microscopy. ATAT1 dramatically changed its localization through the cell cycle, depending on the mitotic phase. In interphase, immunolabeled ATAT1 was observed in centrioles, nuclei, and basal bodies if the cells projected primary cilia. ATAT1 was intensely detected as clusters in the nuclei in the G1–G2 phase. In telophase, ATAT1 colocalized with chromatids and spindle poles, and ultimately migrated to the daughter nucleus, newly synthesized centrioles, and midbody. The nucleolus is a core region of ribosomal RNA transcription, and the midbody is associated with severing and depolymerizing of microtubules in the stembody. The specific distributions of ATAT1 through the cell cycle suggest multiple functions of ATAT1, which could include acetylation of microtubules, RNA transcription activity, severing microtubules, and completion of cytokinesis.





Similar content being viewed by others
References
Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J (2010) MEC-17 is an alpha-tubulin acetyltransferase. Nature 467:218–222
Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV (2010) The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA 107:21517–21522
Janke C, Bulinski JC (2011) Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 12:773–786
L’Hernault SW, Rosenbaum JL (1985) Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24:473–478
Aguilar A, Becker L, Tedeschi T, Heller S, Iomini C, Nachury MV (2014) Alpha-tubulin K40 acetylation is required for contact inhibition of proliferation and cell-substrate adhesion. Mol Biol Cell 25:1854–1866
Kalebic N, Martinez C, Perlas E, Hublitz P, Bilbao-Cortes D, Fiedorczuk K, Andolfo A, Heppenstall PA (2013) Tubulin acetyltransferase alphaTAT1 destabilizes microtubules independently of its acetylation activity. Mol Cell Biol 33:1114–1123
Nakakura T, Asano-Hoshino A, Suzuki T, Arisawa K, Tanaka H, Sekino Y, Kiuchi Y, Kawai K, Hagiwara H (2015) The elongation of primary cilia via the acetylation of alpha-tubulin by the treatment with lithium chloride in human fibroblast KD cells. Med Mol Morphol 48:44–53
Nakakura T, Suzuki T, Nemoto T, Tanaka H, Asano-Hoshino A, Arisawa K, Nishijima Y, Kiuchi Y, Hagiwara H (2016) Intracellular localization of alpha-tubulin acetyltransferase ATAT1 in rat ciliated cells. Med Mol Morphol 49:133–143
Piperno G, LeDizet M, Chang XJ (1987) Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104:289–302
Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21:6820–6831
Tran PT, Joshi P, Salmon ED (1997) How tubulin subunits are lost from the shortening ends of microtubules. J Struct Biol 118:107–118
Maruta H, Greer K, Rosenbaum JL (1986) The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol 103:571–579
Palazzo A, Ackerman B, Gundersen GG (2003) Cell biology: Tubulin acetylation and cell motility. Nature 421:230
Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA (2013) alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat Commun 4:1962
Kim GW, Li L, Gorbani M, You L, Yang XJ (2013) Mice lacking alpha-tubulin acetyltransferase 1 are viable but display alpha-tubulin acetylation deficiency and dentate gyrus distortion. J Biol Chem 288:20334–20350
Li L, Yang XJ (2015) Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell Mol Life Sci 72:4237–4255
Boggs AE, Vitolo MI, Whipple RA, Charpentier MS, Goloubeva OG, Ioffe OB, Tuttle KC, Slovic J, Lu Y, Mills GB, Martin SS (2015) alpha-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res 75:203–215
Nam HJ, Kang JK, Kim SK, Ahn KJ, Seok H, Park SJ, Chang JS, Pothoulakis C, Lamont JT, Kim H (2010) Clostridium difficile toxin A decreases acetylation of tubulin, leading to microtubule depolymerization through activation of histone deacetylase 6, and this mediates acute inflammation. J Biol Chem 285:32888–32896
Castro-Castro A, Janke C, Montagnac G, Paul-Gilloteaux P, Chavrier P (2012) ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol 91:950–960
Valenzuela-Fernandez A, Alvarez S, Gordon-Alonso M, Barrero M, Ursa A, Cabrero JR, Fernandez G, Naranjo-Suarez S, Yanez-Mo M, Serrador JM, Munoz-Fernandez MA, Sanchez-Madrid F (2005) Histone deacetylase 6 regulates human immunodeficiency virus type 1 infection. Mol Biol Cell 16:5445–5454
Husain M, Harrod KS (2011) Enhanced acetylation of alpha-tubulin in influenza A virus infected epithelial cells. FEBS Lett 585:128–132
Naranatt PP, Krishnan HH, Smith MS, Chandran B (2005) Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol 79:1191–1206
Yu CW, Chang PT, Hsin LW, Chern JW (2013) Quinazolin-4-one derivatives as selective histone deacetylase-6 inhibitors for the treatment of Alzheimer’s disease. J Med Chem 56:6775–6791
Zhang L, Liu C, Wu J, Tao JJ, Sui XL, Yao ZG, Xu YF, Huang L, Zhu H, Sheng SL, Qin C (2014) Tubastatin A/ACY-1215 improves cognition in Alzheimer’s disease transgenic mice. J Alzheimers Dis 41:1193–1205
Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F (2007) Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci 27:3571–3583
Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A (2013) Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol Med 5:52–63
Taes I, Timmers M, Hersmus N, Bento-Abreu A, Van Den Bosch L, Van Damme P, Auwerx J, Robberecht W (2013) Hdac6 deletion delays disease progression in the SOD1G93A mouse model of ALS. Hum Mol Genet 22:1783–1790
Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ, De Vos KJ (2014) Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun 5:5245
Wloga D, Gaertig J (2010) Post-translational modifications of microtubules. J Cell Sci 123:3447–3455
Gregory PD, Wagner K, Horz W (2001) Histone acetylation and chromatin remodeling. Exp Cell Res 265:195–202
Muth V, Nadaud S, Grummt I, Voit R (2001) Acetylation of TAF(I)68, a subunit of TIF-IB/SL1, activates RNA polymerase I transcription. EMBO J 20:1353–1362
Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao TP (2002) MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. Embo j 21:6236–6245
Thevenet L, Mejean C, Moniot B, Bonneaud N, Galeotti N, Aldrian-Herrada G, Poulat F, Berta P, Benkirane M, Boizet-Bonhoure B (2004) Regulation of human SRY subcellular distribution by its acetylation/deacetylation. EMBO J 23:3336–3345
Nakakura T, Suzuki T, Torii S, Asano-Hoshino A, Nekooki-Machida Y, Tanaka H, Arisawa K, Nishijima Y, Susa T, Okazaki T, Kiuchi Y, Hagiwara H (2017) ATAT1 is essential for regulation of homeostasis-retaining cellular responses in corticotrophs along hypothalamic-pituitary-adrenal axis. Cell Tissue Res 370:169–178
Okuwaki M, Tsujimoto M, Nagata K (2002) The RNA binding activity of a ribosome biogenesis factor, nucleophosmin/B23, is modulated by phosphorylation with a cell cycle-dependent kinase and by association with its subtype. Mol Biol Cell 13:2016–2030
Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE, Fukasawa K (2000) Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103:127–140
Hunter T, Pines J (1994) Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell 79:573–582
Voit R, Grummt I (2001) Phosphorylation of UBF at serine 388 is required for interaction with RNA polymerase I and activation of rDNA transcription. Proc Natl Acad Sci USA 98:13631–13636
Song Y, Brady ST (2015) Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol 25:125–136
Coombes C, Yamamoto A, McClellan M, Reid TA, Plooster M, Luxton GW, Alper J, Howard J, Gardner MK (2016) Mechanism of microtubule lumen entry for the alpha-tubulin acetyltransferase enzyme alphaTAT1. Proc Natl Acad Sci USA 113:E7176–E7184
Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A (2014) Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157:1405–1415
Satir P, Christensen ST (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129:687–693
Huet XRJ, Plet A, Vié A, Blanchard JM (1996) Cyclin A expression is under negative transcriptional control during the cell cycle. Mol Cell Biol 16:3789–3798
Klein J, Grummt I (1999) Cell cycle-dependent regulation of RNA polymerase I transcription: the nucleolar transcription factor UBF is inactive in mitosis and early G1. Proc Natl Acad Sci USA 96:6096–6101
Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J (2011) Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci USA 108:4846–4851
Hu CK, Coughlin M, Mitchison TJ (2012) Midbody assembly and its regulation during cytokinesis. Mol Biol Cell 23:1024–1034
Steigemann P, Gerlich DW (2009) Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol 19:606–616
Yang D, Rismanchi N, Renvoise B, Lippincott-Schwartz J, Blackstone C, Hurley JH (2008) Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat Struct Mol Biol 15:1278–1286
Bhutta MS, McInerny CJ, Gould GW (2014) ESCRT function in cytokinesis: location, dynamics and regulation by mitotic kinases. Int J Mol Sci 15:21723–21739
Mukai A, Mizuno E, Kobayashi K, Matsumoto M, Nakayama KI, Kitamura N, Komada M (2008) Dynamic regulation of ubiquitylation and deubiquitylation at the central spindle during cytokinesis. J Cell Sci 121:1325–1333
Sudo H, Baas PW (2010) Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci 30:7215–7226
Acknowledgements
We thank Ms. Yuri Amakawa (Teikyo University, Itabashi, Japan) for useful technical support. This work was supported in part by a grant-in-aid for science research from the Ministry of Education, Science, Sports, and Culture of Japan to H.H (Grant No. 17K08523) and by a research Grant from Teikyo University School of Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest that might prejudice the impartiality of this research.
Rights and permissions
About this article
Cite this article
Nekooki-Machida, Y., Nakakura, T., Nishijima, Y. et al. Dynamic localization of α-tubulin acetyltransferase ATAT1 through the cell cycle in human fibroblastic KD cells. Med Mol Morphol 51, 217–226 (2018). https://doi.org/10.1007/s00795-018-0195-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-018-0195-x