Abstract
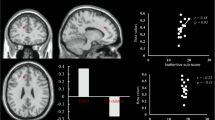
Adults with psychotic disorders have abnormal connectivity of fronto-temporal networks. However, whether these abnormalities are present in adolescents with early psychosis has not been fully assessed. One-hundred and thirty-nine adolescents aged 12–18 underwent resting-state functional magnetic resonance imaging and diffusion tensor imaging. Following motion correction, data were available for 44 participants with a psychosis risk syndrome, 34 patients with a first episode psychosis (FEP) and 35 healthy controls. Independent component analysis was performed to assess functional networks showing a fronto-temporal scope; this identified a language and a salience network. Mean fractional anisotropy was measured in clusters showing between-group differences in intrinsic functional connectivity (iFC). For the language network, there was a group effect within the right middle/inferior frontal gyrus, explained by reduced iFC in patients with an FEP relative to healthy controls, while in participants with a psychosis risk syndrome values of iFC were intermediate. In this region, values of iFC were positively correlated with mean fractional anisotropy in patients with an FEP. No group differences were observed in the salience network. Reduced iFC of the language network, in association with disrupted white matter microstructure, may characterize FEP during adolescence.




Similar content being viewed by others
References
Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK (2005) Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry 162(3):485–494
Plaze M, Bartrés-Faz D, Martinot JL et al (2008) Left superior temporal gyrus activation during sentence perception negatively correlates with auditory hallucination severity in schizophrenia patients. Schizophr Res 87(1–3):109–115
Stephan KE, Friston KJ, Frith CD (2009) Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 35(3):509–527
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711
Raichle ME (2010) Two views of brain function. Trends Cogn Sci 14:180–190
Matthews M, Fair DA (2015) Research review: Functional brain connectivity and child psychopathology–overview and methodological considerations for investigators new to the field. Child Psychol Psychiatry 56(4):400–414
Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A (2011) Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev 35(5):1110–1124
Gavrilescu M, Rossell S, Stuart GW et al (2010) Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol Med 40:1149–1158
Shinn AK, Baker JT, Cohen BM, Ongür D (2013) Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr Res 143:260–268
Calhoun VD, Kiehl KA, Pearlson GD (2008) Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 29(7):828–838
Khadka S, Meda SA, Stevens MC et al (2013) Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry 74(6):458–466
Thoma RJ, Chaze C, Lewine JD et al (2016) Functional MRI EVALUATIOn of Multiple Neural Networks Underlying Auditory Verbal Hallucinations in Schizophrenia Spectrum Disorders. Front Psychiatry 29(7):39
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667
Palaniyappan L, White TP, Liddle PF (2012) The concept of salience network dysfunction in schizophrenia: from neuroimaging observations to therapeutic opportunities. Curr Top Med Chem 12(21):2324–2338
Zhou Y, Liang M, Jiang T et al (2007) Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett 417:297–302
Pu W, Li L, Zhang H et al (2012) Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res 141(1):15–21
Manoliu A, Riedl V, Zherdin A, Mühlau M et al (2014) Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull 40:428–437
Orliac F, Naveau M, Joliot M et al (2013) Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res 148(1–3):74–80
Kraguljac NV, White DM, Hadley JA et al (2015) Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin 22(10):146–158
Berman RA, Gotts SJ, McAdams HM et al (2016) Disrupted sensorimotor and social-cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain 139(Pt 1):276–291
Miller TJ, McGlashan TH, Rosen JL et al (2003) Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 29:703–715
Pettersson-Yeo W, Benetti S, Frisciata S et al (2015) Does neuroanatomy account for superior temporal dysfunction in early psychosis? A multimodal MRI investigation. J Psychiatry Neurosci 40:100–107
Colibazzi T, Horga G, Wang Z et al (2016) Neural Dysfunction in Cognitive Control Circuits in Persons at Clinical High-Risk for Psychosis. Neuropsychopharmacology 41(5):1241–1250
Yoon YB, Yun JY, Jung WH et al (2015) Altered Fronto-Temporal Functional Connectivity in Individuals at Ultra-High-Risk of Developing Psychosis. PLoS One 10(8):e0135347
Wotruba D, Michels L, Buechler R et al (2014) Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophr Bull 40:1095–1104
Wang C, Ji F, Hong Z, Poh JS et al (2016) Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychol Med 46(13):2771–2783
Pelletier-Baldelli A, Bernard JA, Mittal VA (2015) Intrinsic Functional Connectivity in Salience and Default Mode Networks and Aberrant Social Processes in Youth at Ultra-High Risk for Psychosis. PLoS One 10(8):e0134936
Tamnes CK, Agartz I (2016) White Matter Microstructure in Early-Onset Schizophrenia: A Systematic Review of Diffusion Tensor Imaging Studies. J Am Acad Child Adolesc Psychiatry 55(4):269–279
Shergill SS, Kanaan RA, Chitnis XA et al (2007) A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry 164(3):467–473
Oh JS, Kubicki M, Rosenberger G et al (2009) Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp 30(11):3812–3825
Benetti S, Pettersson-Yeo W, Allen P et al (2015) Auditory verbal hallucinations and brain dysconnectivity in the perisylvian language network: a multimodal investigation. Schizophr Bull 41(1):192–200
Leroux E, Delcroix N, Dollfus S (2014) Left fronto-temporal dysconnectivity within the language network in schizophrenia: an fMRI and DTI study. Psychiatry Res 223(3):261–267
Taylor SJ, Barker LA, Heavey L, McHale S (2015) The longitudinal development of social and executive functions in late adolescence and early adulthood. Front Behav Neurosci 15:252
Rubia K (2013) Functional brain imaging across development. Eur Child Adolesc Psychiatry 22(12):719–731
Sole-Padulles C, Castro-Fornieles J, De La Serna E et al (2016) Intrinsic connectivity networks from childhood to late adolescence: effects of age and sex. Dev Cogn Neurosci 17:35–44
Gogtay N, Giedd JN, Lusk L et al (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101(21):8174–8179
Satterthwaite TD, Baker JT (2015) How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol 30:85–91
Satterthwaite TD, Vandekar SN, Wolf DH et al (2015) Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Mol Psychiatry 20(12):1508–1515
Rapado-Castro M, Bartholomeusz CF, Castro-Fornieles J et al (2015) Gender effects on brain changes in early-onset psychosis. Eur Child Adolesc Psychiatry 24(10):1193–1205
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E (2003) The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull 29:633–651
Klosterkötter J, Ruhrmann R, Schultze-Lutter F et al (2005) The European Prediction of Psychosis Study (EPOS): integrating early recognition and intervention in Europe. World Psychiatry 4:161–167
American Psychiatric Association (APA) (1994) Diagnostic and statistical manual of mental disorders (DSM-IV). American Psychiatric Association, Washington
Geller B, Zimerman B, Williams M et al (2001) Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry 40:450–455
Wechsler D (2005) The Wechsler intelligence scale for children IV. TEA ediciones, Madrid
Wechsler D (1999) Wechlser Adult Intelligence Scale III. TEA ediciones, Madrid
Chao-Gan Y, Yu-Feng Z (2010) DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 14(4):13
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen S (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154
Yan CG, Cheung B, Kelly C et al (2013) A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 1(76):183–201
Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD (2011) Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp 32(12):2075–2095
Lui S, Li T, Deng W et al (2010) Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 67:783–792
Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psych 64:663–667
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22:158–165
Friederici AD (2011) The brain basis of language processing: from structure to function. Physiol Rev 91:1357–1392
Seeley WW, Menon V, Schatzberg AF et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 28:2349–2356
Knecht S, Deppe M, Drager B et al (2000) Language lateralization in healthy right-handers. Brain 123:74–81
Simonyan K, Fuertinger S (2015) Speech networks at rest and in action: Interactions between functional brain networks controlling speech production. J Neurophysiol 1:2967–2978
McAvoy M, Mitra A, Coalson RS et al (2016) Unmasking language lateralization in human brain intrinsic activity. Cereb Cortex 26(4):1733–1746
Soroker N, Kasher A, Giora R et al (2005) Processing of basic speech acts following localized brain damage: a new light on the neuroanatomy of language. Brain Cogn 57:214–217
Lee SY, Bang M, Kim KR et al (2015) Impaired facial emotion recognition in individuals at ultra-high risk for psychosis and with first-episode schizophrenia, and their associations with neurocognitive deficits and self-reported schizotypy. Schizophr Res 165:60–65
Crossley NA, Mechelli A, Fusar-Poli P et al (2009) Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp 30(12):4129–4137
Carletti F, Woolley JB, Bhattacharyya S et al (2012) Alterations in white matter evident before the onset of psychosis. Schizophr Bull 38:1170–1179
Fusar-Poli P, Bonoldi I, Yung AR et al (2012) Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 69:220–229
Sugranyes G, Kyriakopoulos M, Dima D et al (2012) Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophr Res 138:136–142
Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15(4):528–536
Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci 113(28):7900–7905
Acknowledgements
The authors would like to thank Mr. Roger Borras for his support with statistical analyses. This work was supported by Grants by the Spanish Ministry of Health, Instituto de Salud Carlos III (PI070066 and PI11/1349) and Fundació La Marató de TV3 (091630). This work has been funded by the project PI11/01349, integrated in the Plan Nacional I+D+I and co-funded by ISCIII-Subdirección General de Evaluación and European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Solé-Padullés, C., Castro-Fornieles, J., de la Serna, E. et al. Intrinsic functional connectivity of fronto-temporal networks in adolescents with early psychosis. Eur Child Adolesc Psychiatry 26, 669–679 (2017). https://doi.org/10.1007/s00787-016-0931-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-016-0931-5