Abstract
Objective
There are 500 million patients living with diabetes mellitus worldwide and 50% of them remain undiagnosed. Routine periodontal probing provides gingival crevicular blood in patients with gingivitis. Gingival blood may be useful for diabetes screening without the need for any expensive, painful or time-consuming method by using convenient glucometers. Therefore, the objective of this systematic review and meta-analysis is to answer the question to “is there a difference in glucose or HbA1c levels (O) in patients with positive gingival bleeding (P) measured on gingival crevicular blood (GCB) (I) compared to finger prick capillary blood (CB) (C).
Materials and methods
The authors performed an electronic search of six databases using identical MeSH phrases. Only human clinical studies without limitations on the year of publication were considered. Data extraction was done by using standardized data collection sheets. Risk of bias assessment were conducted using QUADAS-2 and QUADAS-C. Meta-analyses were carried out with the random effects model to aggregate the correlation coefficients and the difference between the means between gingival and capillary blood reading, using 95% confidence intervals.
Results
The database and manual search yielded 268 articles, from which the selection procedure provided 36 articles for full-text screening, and the final pool of eligible articles composed of 23 studies with 1680 patients. Meta-analysis results on glycemic levels showed differences between the GCB and CB procedures in patients with and without diabetes with values of -6.80 [-17.35; 3.76] and − 4.36 [-9.89; 1.18], respectively. Statistically significant correlations were found (p = 0.001) between GCB and CB measurements in patients with (0.97 [0.927; 0.987]) and without diabetes (0.927 [0.873; 0.958]).
Conclusion
Gingival blood could prove to be useful to identify patients with undiagnosed diabetes when the necessary amount of uncontaminated blood is present. However, this technique is limited by the possibility of contamination, prandial status and inaccuracies, so it is unsuited to address the patient’s glycemic control accurately.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is known to be one of the major global epidemic diseases with more than 500 million patients worldwide of which 50% are undiagnosed cases. It is significantly associated with mortality and morbidity, conferring a substantial burden to the healthcare system with an approximate of USD 960 billion dollars in expenditure [1, 2]. DM is a chronic metabolic disorder that leads to hyperglycemia, which raises multiple complications caused by micro- and macroangiopathy [3]. Chronic hyperglycemia leads to increased pro-inflammatory cytokine levels both systematically and locally which leads to increased occurrence of periodontitis, significant risk of tooth loss, delayed wound healing and impaired response to infections [4, 5]. Moreover, poorly controlled DM increases the risk and severity of periodontitis, peri-implantitis, and diminishes the effectivity of periodontal treatment therapy [6]. However, there is still no evidence that dental implant surgery is contraindicated in patients with prediabetes or well-controlled DM [7]. Therefore, screening patients for undiagnosed DM, and also checking the quality of glycemic control is an important aspect for dental surgeries.
There are four ways of diagnosing DM according to the American Diabetes Association, HbA1c level, fasting plasma glucose (FPG), oral glucose tolerance test (OGTT) and random plasma glucose test (RPG). OGTT is impractical at the average dental setting, while FPG is only suggested when non-invasive procedures are planned, for eight hours of fasting are required before measuring. For HbA1c, values higher than 6.5% (48 mmol/mol) indicate DM, in case of FPG measurement the value is 6.9 mmol/L (125 mg/dl), whereas for RPG only severe DM can be detected with values higher than 11.1 mmol/L (200 mg/dl) [8].
The U.S. Preventive Services Task Force determined there is sufficient evidence that lifestyle interventions can prevent or delay progression to type II DM [9]. Moreover, early diagnosis of DM is key to avoid the microvascular consequences of the disease since approximately 25% of newly diagnosed patients have already developed at least one complication [10]. Appropriate screening devices and standardized methods are crucial to prevent this potentially inauspicious life condition. Dental teams can assist in the early detection, diagnosis and treatment of DM and, secondarily, other chronic conditions, such as cardiovascular disease [11].
Routine periodontal probing produces gingival crevicular blood (GCB) in patients with gingivitis or periodontitis. In recent years, some published clinical studies showed that the GCB may be useful for DM screening without the need for any extra and uncomfortable procedure like the need for finger puncture with sharp lancets. Currently, the glucometer is the conventional device employed for capillary finger-stick blood glucose level determination which can be also used to measure the glucose content of gingival blood [12,13,14]. Routine probing during a periodontal examination is more familiar to the practitioners and less traumatic for the patients. Plasma HbA1c levels represent the last two to three months of average systemic blood glucose levels, which gives additional insight on glycemic control besides direct blood glucose measurements [15]. Even in the cases of low gingival crevicular bleeding, a glucose measurement is possible with the help of the self-monitoring device. In addition, the sampling procedure is much easier to perform and less time-consuming [16].
To the best of our knowledge, there are no systematic reviews published in the literature in this regard. Hence, the aim of this study is to assess the reliability of using gingival crevicular blood for identifying patients with undiagnosed DM and assessing their quality of glycemic control in the dental setting. Moreover, we aim to interpret the probable variations in the results obtained by the researchers who have examined the feasibility and acceptability of using gingival crevicular blood as an alternative to capillary blood (CB) to measure blood glucose and HbA1c levels.
Material & methods
Search strategy
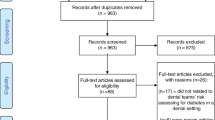
This systematic review was performed according to the Cochrane Handbook and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement items (Fig. 1.) [17]. We also developed and submit a protocol to the International Prospective Register of Systematic Reviews (PROSPERO) database (ID: CRD42022372748).
We applied the literature search to answer the following focused question: is gingival crevicular blood a reliable tool to measure HbA1c and glucose levels in the dental setting?
In this regard, the following PICO framework was used:
-
Participants (P) are the adult individuals with positive bleeding on probing.
-
The intervention (I) is collecting the gingival crevicular blood and using a glucometer to estimate the blood glucose level.
-
The comparison (C) is using other sources of blood to measure the blood glucose level.
-
The Outcome (O) is measuring Hemoglobin A1c and glucose level.
Data sources & search strategy
We performed an electronic literature search using a wide range of computerized databases, including MEDLINE, Cochrane Library, PsycINFO, Web of Science, Google Scholar and Scopus on 15 July 2023. We did not use any filters based on language or publication date in our electronic literature search. We used the following search terms and protocols in this systematic review:
((gingival crevicular blood) OR (crevicular blood)) AND ((((((((Diabetes mellitus) OR (Hyperglycemia)) OR (High blood glucose levels)) OR (type 2 diabetes)) OR (Finger stick blood)) OR (Finger prick blood)) OR (Glucometer)) OR (glucose)) OR (hemoglobin A1c)).
The terms and keywords were adapted for each database as necessary. We also performed an extensive manual search encompassing the bibliographies and citations of the included papers and review articles. Furthermore, we searched the websites that list ongoing clinical trials: (http://clinicaltrials.gov, http://www.centerwatch.com/http://www.clinicalconnection.com).
Eligibility criteria
The inclusion criteria for this study were as follows: (1) Human clinical studies comprising randomized controlled trials, prospective studies, retrospective studies, and case series; (2) The investigations involved collecting the gingival crevicular blood and at least one other blood sample to measure hemoglobin A1c and/or glucose levels; (4) a minimum of twenty patients; (6) no deadline for publication date.
The exclusion criteria were as follows: (1) nonclinical and animal studies, case reports, review articles, and commentaries (2) the unavailability of full-text articles; and (5) full-text papers written in a language other than English.
Study selection & data extraction
At the first study selection step, two reviewers (OF & MP) independently screened the (1) titles and (2) abstracts. Subsequently, the full text of all eligible studies was obtained and checked by the same reviewers [17]. Disagreements were resolved through discussion. After that, we excluded the publications that did not meet the eligibility criteria and we recorded the reasons for exclusion.
Afterwards we extracted and assimilated data on a piloted, standardized data collection sheet. We classified all the data in relation to year of publication, country, measurement methods, patient characteristics, confounding factors and outcomes according to the aims of this study.
Quality assessment
Two reviewers (OF & MP) independently conducted a risk of bias assessment using QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies-2) and QUADAS-C (Quality Assessment of Diagnostic Accuracy Studies–Comparative instruments [18, 19].
The QUADAS-C tool can assess risk of bias in test comparisons undertaken in comparative accuracy studies. QUADAS-C is an extension of QUADAS-2 [18]. The QUADAS tool was used to assess risk of bias and concerns regarding patient selection, index test, reference standard, and flow and timing, with each domain being classified into one of three categories: (i) high risk of bias; (ii) unclear risk of bias; and (iii) low risk of bias. T [19, 20]. Any discrepancy between reviewers in quality ratings was resolved by discussion and consensus.
In addition, we assigned a level of evidence for each article using the classification system described by Wright et al. [21].
Statistical analysis
The meta-analysis was conducted using R software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria) to pool correlation coefficients and to calculate the mean differences (MD) [22]. The analysis was conducted by using the random effects model assuming significant between-study heterogeneity. To calculate the difference between the means, continuous variables, and 95% confidence intervals were used. To calculate the study MDs and pooled MD, the sample size, the mean and the corresponding standard deviation (SD) was extracted from each study (in each group separately). We reported the results as the experimental group minus the control group values. Subsequently, the Pearson correlation coefficients from the included studies were pooled and analyzed, forest plots were created for both analyses [23]. This was carried out once for patients with DM and once for patients without DM independently. Heterogeneity across the studies was assessed using the I2 test [24]. An I2 value greater than 75% was considered high.
Results
Study selection
A total of 268 possibly relevant articles were identified through the search strategy. After completing the screening titles and eliminating duplicates, 131 studies were retrieved, and their abstract versions were collected for further assessment (Kappa value = 0.87). We selected Thirty-six studies based on the abstract screening phase. A manual search for the reference lists of the 36 studies revealed no additional qualifying paper. Then we assessed the full text version of these 36 studies. According to the results of the full article review stage, thirteen articles were excluded. The reasons for excluding full-text articles are presented in Fig. 2. PRISMA flow chart of selection process.
Finally, we included 23 eligible clinical studies to this systematic review and meta-analysis study according to predefined inclusion and exclusion criteria.
Study characteristics
The included articles were published between 1993 and 2023. The majority of the literature is from India with 13 studies [13, 25,26,27,28,29,30,31,32,33,34,35], two from Kuwait [36, 37] and the USA [38, 39], and one from China [14], Italy [12], Pakistan [40], Jordan [41], Germany [42] and Iran [43] respectively. All in all, the studies included 1680 mostly middle-aged patients with an average age of 44.4 and with a roughly equal distribution of sex with slightly more females (51%). The basic characteristics of the included studies can be found in Table 1.
Risk of bias and level of evidence in studies
Based on the QUADAS-C risk of bias tool, the included studies received low risk of bias, only five studies missed on reporting the time between index and reference test, although it did not affect overall risk of the studies (Fig. 3). According to the classification system described by Wright et al., we assigned level III evidence for all the included articles. Referring to this classification system under the diagnostic research category, studies of nonconsecutive patients (without consistently applying the reference gold standard) should be considered as level III evidence.
Results of individual studies
Only one study has reported on HbA1c level measurements in patients with and without DM in severe and moderate periodontitis. The GCB and CB values in patients with DM were 7.72% ± 1.71%, and 7.89% ± 1.78%, while in patients without DM, the values were 5.28% ± 0.3%, and 5.23% ± 0.32%, respectively. There were highly significant correlations between the measurements with values of r = 0.977, and r = 0.829, respectively [14]. Due to the insufficient number of studies, performing quantitative analysis on HbA1c measurements was not possible.
18 studies have reported on the specific mean glucose levels measured from GCB and capillary blood (CB) with 12 different glucometers, the most frequently used glucometer was Accu-Check in 4 studies. 16 studies have reported their outcomes in mg/dl while two in mmol/l which were converted to mg/dl by the authors. The highest and lowest mean values recorded from GCB was 243.27 and 156.07 mg/dl in the DM group and 118.76 and 90.08 mg/dl in the non-DM group respectively [12, 25,26,27,28,29,30,31,32,33,34,35, 37, 40, 41, 43,44,45]. Two of the studies have found statistically significant differences between GCB and CB values (p = 0.001) [33, 37]. The first study’s mean values for the DM group were 210.56 ± 17.26 mg/dl 178.08 ± 17.66 mg/dl and for the non-DM group 118.76 ± 13.83 mg/dl 86.56 ± 10.17 mg/dl respectively [33]. The second study reported a mean value of 77.94 ± 38 mg/dl for GCB and 102.96 ± 37 mg/dl for CB [37].
One study has reported on the exact periodontal status of the patients as gingival indexes and probing depths. Patients with DM had higher gingival index and probing depth values compared to non-DM patients with 2.18 ± 0.39 and 4.43 ± 0.97 mm against 1.77 ± 0.28 and 3.96 ± 0.75 mm respectively [29].
Results of synthesis
Meta-analysis was performed to calculate the differences between the mean values of blood glucose level measurements between GCB and CB sampling sites in patients with and without DM. The analysis did not yield statistically significant differences with values of -6.80 [-17.35; 3.76] and − 4.36 [-9.89; 1.18] in patients with and without DM, respectively. The heterogeneity of the analysis remained low in the DM patient group with an I2 value of 36%, whereas high heterogeneity was detected in the non-DM patient group (I2 = 80%) (Fig. 4).
Subsequently, quantitative analysis was performed on the Pearson’s correlations between GCB and CB glucose level values in DM and non-DM patients’ groups (Figs. 5 and 6.). 16 and 12 studies have reported on the necessary data for non-DM and DM patient group values respectively. Statistically significant (p = 0.001) correlations were found for the DM using the random effects model with a value of 0.97 [0.927; 0.987] using 95% confidence intervals with substantial heterogeneity I2 = 91,5%. Similar statistically significant (p = 0.001) results were found for the non-DM patient groups with a value of 0.927 [0.873; 0.958] with substantial heterogeneity I2 = 93.2%.
Discussion
A systematic review and meta-analysis were performed on the reliability of GCB glucose and HbA1c measurement to assess glycemic control and identify patients with DM. The meta-analysis has found statistically significant correlations between GCB and CB in both DM and non-DM patient groups.
In order to assess a patient’s chronic glycemic control HbA1c is the easiest and most important value to measure, hence It quantifies the last two to three months of average blood glucose levels. However only assessing a patient’s glycemic control based on HbA1c can be misleading, since a given HbA1c value can be associated with wide ranges of mean glucose values, therefore knowing the patients current mean glucose level can help to interpret the meaning of actual HbA1c levels [46]. Only one study by Wu et al. have compared the HbA1c values from GCB to CB using an ion-exchange high-performance liquid chromatography where they have found highly significant correlations even with elevated HbA1c levels in patients with different degrees of periodontitis. In order to confirm the appropriateness of HbA1c level measurements from GCB additional studies are required [44].
Chair-side glucose meters are an increasingly popular option to assess average blood glucose levels in a time [47]. A study conducted by Ekhlaspour et al. in 2016 have compared the accuracy of 17 widely available glucometers to ISO standards and only 7 and 2 glucometers had the diagnostic accuracy to meet the ISO 2003 and 2013 standards respectively [48]. The U.S. Food and Drug Administration have updated the requirements for diagnostic accuracy in 2019 with requirements of 95% within +/- 15% across the measuring range and 99% within +/- 20% across the measuring range, therefore the use of glucometers with the updated FDA regulations are highly advised to gather correct glucose readings [49].
Most of the examined studies are supporting the use of GCB to measure blood glucose levels. The main advantages of using GCB as the source is the time-effectiveness that it can be done during routine periodontal examination by the dentist while not requiring to wait long times for the results [12]. Secondly, the cost-effectiveness of the procedure plays a major role in the wide-scale usability of this technique, since the purchase of glucometers and test-strips are very modest [39]. Thirdly the patient’s comfort is an important aspect of the procedure hence there is no need for an additional finger puncture therefore blood glucose measurements can be done without any pain or inconvenience [42]. Even though our analysis has found significant correlations between the results of the different sampling sites, we also found differences between the means that are not statistically significant, but in certain cases can prove to be clinically relevant. These differences are larger in patients with DM compared to patients without DM. Therefore, this procedure is unsuited to accurately assess glycemic control in patients with DM, however it may be useful to screen undiagnosed cases. According to the ADA, FBG is below 130 mg/dL in patients without DM, and the majority of patients have values between 70 and 100 mg/dL. Hence, GCB can possibly be useful to detect blood glucose levels in the DM range in the majority of the population despite the differences. Although, it is important to note, when high glucose levels are present, the readings should be confirmed by conventional CB measurements as well. Unfortunately, the prandial status significantly affects the patient’s glycemic levels at the time of measurement, and it proves to be a significant limitation of this procedure [50]. To overcome this, measuring HbA1c level would be more prominent, since it is unaffected by the patient’s prandial status, and can provide a more accurate view on the patient’s overall systemic glycemic load of the last two to three months [51]. Due to the very limited amount of evidence on HbA1c level measurements from GCB, it is not possible to draw any definitive conclusions, but the results are promising, and more prospectively designed studies are necessary to allow for quantitative analysis. One of the most important limitations is that gingival inflammation is necessary to be present in order to obtain sufficient amount of GCB for the test strip after the periodontal examination, since elevated bleeding is usually present with advanced inflammation. Most available glucometers need at least 4 µL blood to give correct readings [12]. Three studies concluded that GCB cannot be used as a source to measure blood glucose levels. Müller et al. have received error readings in every 3rd case due to the low amounts of GCB and found significant differences between GCB and CB measurements. These differences may have been caused by the low GCB volumes diluted by gingival fluids [36, 37]. The study conducted by Debnath et al. has found statistically significant differences between GCB and CB and found very low correlation values. In that study GCB readings were consistently higher compared to CB readings, which may have been because of lower amounts of gingival blood and higher amounts of gingival fluids in the source of measurement. The lower amounts of GCB could have been present because of the inclusion of patients with very mild cases of periodontitis [33].
There have been several outliers in our analysis on the correlation between GCB and CB readings which have caused high heterogeneity in the analysis. Low correlations between readings could be explained in several studies by the low amounts of GCB and possible contaminations by gingival fluid [33, 35, 36]. In the study conducted by Bhavsar et al. lower correlation values were found only in the non-DM patient group, which could be explained by the significantly lower GI in the non-DM groups, therefore GCB amounts may have been lower in the non-DM group [29]. Strauss et al. have compared the correlations of GCB and CB measurements and found significant differences between patients with milder and more severe periodontitis. Patient groups with smaller probing depths have presented lower correlation values, the cause for this discrepancy could be answered with the unsatisfying amount of GCB which may result in imprecise readings [39].
Strengths and limitations
The strength of our study is that we could include high number of studies for both quantitative and qualitative analysis and it is the first systematic review on this topic to the best of our knowledge. Our limitation is that in our analysis the heterogeneity remained very high which could be explained by the different glucometers used, different types and severity of DM and periodontitis in the patient groups. Unfortunately, this issue prevented us from presenting more thorough analyses of the blood glucose and HbA1c values reported by the included studies as well as some further analysis, including comparing concordance, or measurement repeatability. The last but not least limitation of our study is the level of evidence in the included studies (level III) that warrants cautious conclusions.
Implications for future research
In order to increase the certainty of evidence provided by clinical studies on the topic studies with more rigorous methodologies are required. The type of glucometers used and the severity of periodontal inflammation heavily influences the accuracy of readings, therefore the use of FDA approved glucometers and similar periodontal status matched cohorts are highly suggested for future studies. More studies are required to assess the accuracy of GCB readings regarding HbA1c levels. HbA1c gives an overview of patient blood glucose levels of the last two to three months, therefor it is widely used of DM diagnosis.
Conclusion
We have found that gingival crevicular blood could be used to measure blood glucose levels to identify patients with undiagnosed diabetes, if the necessary amount of uncontaminated gingival blood is present for a correct reading. However, the procedure is unsuited to monitor glycemic control in patients with diabetes, due to the higher inaccuracies in elevated glucose levels. When a glucose level in the hyperglycemia range (> 130 mg/dL) is detected in a patient without diabetes, conventional finger prick blood measurement is advised for validation. It is important to emphasize the use of FDA approved glucometers in order to ensure the accuracy of all measurements.
Clinical relevance
Scientific rationale for study
Diabetes mellitus is a well-known risk factor of developing severe periodontitis. More than half of the patients living with diabetes remain undiagnosed. The link between periodontitis and diabetes makes periodontal screening a perfect opportunity to identify patients with underlying diabetes. There is no previous comprehensive review of the available literature on the topic of investigating the reliability of gingival crevicular blood on blood glucose and HbA1c level measurements.
Principal findings
We have found statistically significant correlations between gingival crevicular blood and capillary blood glucose measurements, and statistically insignificant, but clinically relevant differences between the mean values.
Practical implications
When a necessary amount of gingival blood is present, an FDA approved glucometer can be used to screen for elevated blood glucose levels in patients without diabetes. In case of post prandial measurement, higher than 140 mg/dL value suggests that hyperglycemia is present, and conventional finger prick testing is necessary to verify the results. It is important to note, that due to the low quality of the studies, the certainty of evidence remains low.
Data availability
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
References
Willey VJ, Kong S, Wu B, Raval A, Hobbs T, Windsheimer A, Deshpande G, Tunceli O, Sakurada B, Bouchard JR (2018) Estimating the real-world cost of diabetes mellitus in the United States during an 8-year period using 2 cost methodologies. Am Health drug Benefits 11:310
Magliano D, Boyko EJ (2021) IDF Diabetes Atlas. International Diabetes Federation
Mauricio D, Alonso N, Gratacòs M (2020) Chronic diabetes complications: the need to move beyond classical concepts. Trends Endocrinol Metabolism 31:287–295
Miller A, Ouanounou A (2020) Diagnosis, management, and dental considerations for the diabetic patient. J Can Dent Assoc 86:1488–2159
Végh D, Bencze B, Banyai D, Vegh A, Rózsa N, Nagy Dobó C, Biczo Z, Kammerhofer G, Ujpal M, Díaz Agurto L, Pedrinaci I, Peña Cardelles JF, Magrin GL, Padhye NM, Mente L, Payer M, Hermann P (2023) Preoperative HbA1c and blood glucose measurements in diabetes Mellitus before oral surgery and implantology treatments. Int J Environ Res Public Health 20. https://doi.org/10.3390/ijerph20064745
Graves DT, Ding Z, Yang Y (2020) The impact of diabetes on periodontal diseases. Periodontology 2000 82:214–224
Jiang X, Zhu Y, Liu Z, Tian Z, Zhu S (2021) Association between diabetes and dental implant complications: a systematic review and meta-analysis. Acta Odontol Scand 79:9–18
Committee ADAPP (2021) 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes—2022. Diabetes Care 45:S17–S38. https://doi.org/10.2337/dc22-S002
Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M (2021) Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA 326:736–743
Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K (2020) Microvascular complications of type 2 diabetes Mellitus. Curr Vasc Pharmacol 18:117–124. https://doi.org/10.2174/1570161117666190502103733
Yonel Z, Batt J, Jane R, Cerullo E, Gray LJ, Dietrich T, Chapple I (2020) The role of the oral healthcare team in identification of type 2 diabetes mellitus: a systematic review. Curr Oral Health Rep 7:87–97
Rapone B, Ferrara E, Santacroce L, Topi S, Converti I, Gnoni A, Scarano A, Scacco S (2020) Gingival Crevicular Blood as a potential Screening Tool: A Cross Sectional comparative study. Int J Environ Res Public Health 17:7356
Sande AR, Guru S, Guru R, Gaduputi S, Thati DK, Siddeshappa ST (2020) Gingival Crevicular blood glucose levels. Is it a Reliable Tool for Screening Diabetes in a Dental Office? J Contemp Dent Pract 21:421–425
Wu J, Lin L, Zhang R, Liu S, Sun W (2021) Can gingival crevicular blood effectively screen for diabetes in Chinese patients with moderate to severe periodontitis? A pilot study. J Dent Sci 16:1–6
(2011) WHO Guidelines Approved by the Guidelines Review Committee. Book title. World Health Organization. Copyright © World Health Organization 2011., Geneva
Venkatesan AN, Amaldas J, Valiathan M, Jayaram V, Jayaraman P (2018) Two different methods for Collection of Gingival Crevicular blood to Estimate Random blood glucose Levels—A comparative study. J Orofac Sci 10:117
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, Deeks JJ, Leeflang MM, Group†, Q-C (2021) QUADAS-C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med 174:1592–1599
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, Group* Q- (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536
Dinnes KSG, Jones HE, Hyde CJ, Lange S, Langendam MW, Leeflang MM, Macaskill P, Mallett S, McInnes MD, Reitsma JB (2021) Guidance on how to use. QUADAS-C. Book title.
Wright JG, Swiontkowski MF, Heckman JD (2003) Introducing levels of evidence to the journal. J Bone Joint Surg Am 85:1–3
R Development Core Team (2010) R; a language and enviroment for statistical computing. Book title. R Foundation for Statistical Computing, Vienna, Austria
Benesty J, Chen J, Huang Y, Cohen I (2009) Pearson Correlation Coefficient. In: Cohen I, Huang Y, Chen J, Benesty J (eds) Book title. Springer Berlin Heidelberg, Berlin, Heidelberg
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Rajesh KS, Irshana R, Arun Kumar MS, Hegde S (2016) Effectiveness of glucometer in screening diabetes mellitus using gingival crevicular blood. Contemp Clin Dent 7:182–185. https://doi.org/10.4103/0976-237x.183072
Sibyl S, Bennadi D, Kshetrimayum N, Manjunath M (2017) Correlations between gingival crevicular blood glucose and capillary blood glucose: a preliminary report. J Lab Physicians 9:260–263. https://doi.org/10.4103/jlp.Jlp_141_16
Gaikwad S, Jadhav V, Gurav A, Shete AR, Dearda HM (2013) Screening for diabetes mellitus using gingival crevicular blood with the help of a self-monitoring device. J Periodontal Implant Sci 43:37–40. https://doi.org/10.5051/jpis.2013.43.1.37
Shetty N, Shankarapillai R, Mathur LK, Manohar B, Mathur A, Jain M (2013) Gingival crevicular blood: as a non-invasive screening tool for diabetes mellitus in dental clinics. J Indian Soc Periodontol 17:472–477. https://doi.org/10.4103/0972-124x.118319
Bhavsar M, Brahmbhatt N, Sahayata V, Bhavsar N (2016) Gingival crevicular blood for screening of blood glucose level in patients with & without diabetes: a chair-side test. Int J Dental Hygiene 14:92–97. https://doi.org/10.1111/idh.12139
Shylaja MD, Punde PA, Sam G, Khan SN, Latheef AA, Thorat AJ (2016) Noninvasive technique for estimating blood glucose levels among Diabetic patients. J Contemp Dent Pract 17:248–252. https://doi.org/10.5005/jp-journals-10024-1835
Parihar S, Tripathi R, Parihar AV, Samadi FM, Chandra A, Bhavsar N (2016) Estimation of gingival crevicular blood glucose level for the screening of diabetes mellitus: a simple yet reliable method. J Oral Biol Craniofac Res 6:198–203. https://doi.org/10.1016/j.jobcr.2016.05.004
Kaur H, Singh B, Sharma A (2013) Assessment of blood glucose using gingival crevicular blood in diabetic and non-diabetic patients: a chair side method. J Clin Diagn Res 7:3066–3069. https://doi.org/10.7860/jcdr/2013/7705.3854
Debnath P, Govila V, Sharma M, Saini A, Pandey S (2015) Glucometric assessment of gingival crevicular blood in diabetic and non-diabetic patients: a randomized clinical trial. J Oral Biol Craniofac Res 5:2–6. https://doi.org/10.1016/j.jobcr.2014.12.004
Dwivedi S, Verma S, Shah M, Jain K (2014) Can gingival crevicular blood be relied upon for assessment of blood glucose level? N Y State Dent J 80:38–42
Gupta A, Gupta N, Garg R, Jain N, Atreja G, Walia SS (2014) Developing a chair side, safe and non-invasive procedure for assessment of blood glucose level using gingival crevicular bleeding in dental clinics. J Nat Sci Biol Med 5:329–332. https://doi.org/10.4103/0976-9668.136177
Müller HP, Behbehani E (2005) Methods for measuring agreement: glucose levels in gingival crevice blood. Clin Oral Investig 9:65–69. https://doi.org/10.1007/s00784-004-0290-3
Müller HP, Behbehani E (2004) Screening of elevated glucose levels in gingival crevice blood using a novel, sensitive self-monitoring device. Med Princ Pract 13:361–365. https://doi.org/10.1159/000080474
Parker RC, Rapley JW, Isley WL, Spencer PJ, Killoy WJ (1993) Gingival crevicular blood for assessment of blood glucose in diabetic patients. J Periodontol 64 7:666–672
Strauss SM, Wheeler AJ, Russell SL, Brodsky A, Davidson RM, Gluzman R, Li L, Malo RG, Salis B, Schoor R, Tzvetkova K (2009) The potential use of gingival crevicular blood for measuring glucose to screen for diabetes: an examination based on characteristics of the blood collection site. J Periodontol 80:907–914. https://doi.org/10.1902/jop.2009.080542
Saeed Q, Memon S, Hosein M, Ahmed A, Ikram S (2021) Correlation between glycaemic state and tooth mobility in patients with periodontal disease. J Pak Med Assoc 71:1337–1340. https://doi.org/10.47391/jpma.1367
Khader Y, Al-Zu’bi B, Judeh A, Rayyan M (2006) Screening for type 2 diabetes mellitus using gingival crevicular blood. Int J Dental Hygiene 4:179–182. https://doi.org/10.1111/j.1601-5037.2006.00206.x
Beikler T, Kuczek A, Petersilka G, Flemmig TF (2002) In-dental-office screening for diabetes mellitus using gingival crevicular blood. J Clin Periodontol 29:216–218. https://doi.org/10.1034/j.1600-051x.2002.290306.x
Ardakani MR, Moeintaghavi A, Haerian A, Ardakani MA, Hashemzadeh M (2009) Correlation between levels of sulcular and capillary blood glucose. J Contemp Dent Pract 10:10–17
Wu J, Lin L, Zhang R, Liu S, Sun W (2021) Can gingival crevicular blood effectively screen for diabetes in Chinese patients with moderate to severe periodontitis? A pilot study. J Dent Sci 16:1–6. https://doi.org/10.1016/j.jds.2020.08.013
Patel C, Dave B, Patel R, Kumar S, Dattani V, Joshi S, Haque M (2023) Gingival Crevicular Blood glucose as a Novel Method for Screening Diabetes Mellitus in Periodontally compromised patients. Cureus 15:e39444. https://doi.org/10.7759/cureus.39444
Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM (2017) The fallacy of average: how using HbA(1c) alone to assess Glycemic Control can be misleading. Diabetes Care 40:994–999. https://doi.org/10.2337/dc17-0636
Bala Raghavendra GN, Bhat SG (2010) Glucometer as a chairside device to assess blood glucose in periodontal patients. J Int Clin Dent Res Organ 2:130–135. https://doi.org/10.4103/2231-0754.95286
Ekhlaspour L, Mondesir D, Lautsch N, Balliro C, Hillard M, Magyar K, Radocchia LG, Esmaeili A, Sinha M, Russell SJ (2017) Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol 11:558–566. https://doi.org/10.1177/1932296816672237
Katz LB, Stewart L, King D, Cameron H (2020) Meeting the New FDA Standard for Accuracy of Self-Monitoring blood glucose Test systems intended for Home Use by Lay users. J Diabetes Sci Technol 14:912–916. https://doi.org/10.1177/1932296820906184
Kaur B, Koh M, Ponnalagu S, Henry CJ (2020) Postprandial blood glucose response: does the glycaemic index (GI) value matter even in the low GI range? Nutr Diabetes 10:15. https://doi.org/10.1038/s41387-020-0118-5
Ghazanfari Z, Haghdoost AA, Alizadeh SM, Atapour J, Zolala F (2010) A comparison of HbA1c and fasting blood Sugar tests in General Population. Int J Prev Med 1:187–194
Acknowledgements
Not applicable.
Funding
Not applicable.
Open access funding provided by Semmelweis University.
Author information
Authors and Affiliations
Contributions
O, F; M, P: conceptualization, project administration, methodology, formal analysis, writing – original draft; B,B: conceptualization, data curation, writing - review & editing; D,V: conceptualization, writing - review & editing; I,M: statistical analyses; All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest relevant to this article were reported by the authors.
Statement of clinical relevance
• From more than 500 million patients with diabetes mellitus 50% are undiagnosed.
• Diabetes mellitus is a significant risk factor of periodontitis.
• Using Gingival crevicular blood provided on routine periodontal probing may prove to be a great opportunity to identify underlying diabetes mellitus.
• This method could be less unpleasant, alternative with less pain and discomfort for the patient.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fakheran, O., Bencze, B., Mischak, I. et al. The reliability of using gingival crevicular blood to measure blood glucose and hba1c levels in the dental setting: a systematic review and meta-analysis. Clin Oral Invest 28, 299 (2024). https://doi.org/10.1007/s00784-024-05685-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05685-4