Abstract
Objective
Craniofacial anomalies are widely discussed as predisposing factors of breathing disorders. Since many more cofactors exist, this study investigated the association between maxillary micrognathia and morphological changes of posterior airway space and adenoids in these patients.
Material and methods
Cephalometric radiographs of n = 73 patients were used for data acquisition. The patients were divided into two groups according to certain skeletal characteristics: maxillary micrognathia (n = 34, 16 female, 18 male; mean age 10.55 ± 3.03 years; defined by a SNA angle < 79°) and maxillary eugnathia (n = 39, 19 female, 20 male; mean age 10.93 ± 3.26 years; defined by a SNA angle > 79°). The evaluation included established procedures for measurements of the maxilla, posterior airway space and adenoids. Statistics included Kolmogorov–Smirnov-, T- and Mann–Whitney-U-Tests for the radiographs. The level of significance was set at p < 0.05.
Results
The cephalometric analysis showed differences in the superior posterior face height and the depth of the posterior airway space at palatal level among the two groups. The depth of the posterior airway space at mandibular level was the same for both groups, just as the size of the area taken by adenoids in the nasopharynx.
Conclusions
Skeletal anomalies affect the dimension of the posterior airway space. There were differences among the subjects with maxillary micrognathia and these with a normal maxilla. However, the maxilla was only assessed in the sagittal direction, not in the transverse. This study showed that the morphology of the maxilla relates to the posterior airway space whereas the adenoids seem not to be affected.
Clinical relevance
Maxillary micrognathia is significantly associated with a smaller depth of the posterior airway space at the palatal level compared to patients with maxillary eugnathia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tube-shaped pharynx consists of three parts: nasopharynx, oropharynx and hypopharynx, starting at the cranial base and ending at the sixth cervical vertebra. An open upper airway is indispensable for nasal breathing and improves growth of craniofacial structures [18]. Therefore, it is of great interest for orthodontists, pediatricians, ENT specialists and speech therapists. Respiratory function and its impact on craniofacial growth have been investigated even more controversially. Because of the close relationship between the posterior airway space and the craniofacial structures an interaction must occur. Obstruction of the upper airway is a predisposing factor for breathing problems [16]. Pharyngeal dimensions are associated with craniofacial anomalies such as maxillary or mandibular micrognathia [1]. Class II malocclusions are seen as consequence of a posterior position of the tongue impacting the cervical region and its respiratory function resulting in mouth breathing or wrong deglutition [2]. For treatment planning pharyngeal morphology is useful to be taken in consideration with the orthodontic diagnosis. Sorensen et al. [24] described the relationship of the mandible and the posterior airway space as more important than the one of the maxilla. Ceylan et al. [2] controverted this relationship.
Aims of the study
Since many different conclusions of craniofacial anomalies and their relationship with the pharyngeal dimension coexist, this study investigated the influence of the maxillary relationship on the depth of the posterior airway space and the adenoids. Therefore, research was restricted to growing patients without any orthodontic treatment only, because the morphology of the maxilla changes due to orthodontic appliances. The use of landmarks on cephalometric radiographs should be verified as a probable method to analyze the maxillary position and dimension of the posterior airway space as well as the size of the area taken by the adenoids.
Material and methods
Patients
The patients were divided into two groups (maxillary micrognathia and maxillary eugnathia), and compared to each other. Cephalometric radiographs of 73 non-syndromic patients (34 maxillary micrognathia, 39 maxillary eugnathia – including 7 with maxillary prognathia) at the age of 10.55 ± 3.03 years (maxillary micrognathia) and 10.93 ± 3.26 years (maxillary eugnathia) were retrospectively identified and analyzed. All patients were exclusively diagnosed for orthodontic treatment at Saarland University Hospital.
Inclusion/Exclusion criteria
The presence of maxillary micrognathia (SNA angle < 79°) and mandibular eugnathia (SNB angle < 81°, ANB angle < 0°) for group 1 (n = 34) and maxillary eugnathia or prognathia (SNA angle > 79°) and mandibular eugnathia or retrognathia (SNB angle < 81°, ANB angle > 0°) for group 2 (n = 39) were the inclusion criteria. The limit for SNA angle for maxillary eugnathia was set at 79° to 83°. The limit for SNB angle for mandibular eugnathia was set at 77° to 81° [6]. Exclusion criteria included mandibular macrognathia, comorbid syndromes and genetic disorders.
As a precondition, diagnostic data including digital cephalometric radiographs had to be present. Data were extracted from before the beginning of orthodontic treatment at the age of ten to thirteen or rather fourteen years.
Control group
All patients with maxillary micrognathia (n = 34) were matched with patients with maxillary eugnathia (n = 39, including the 7 patients with maxillary prognathia). The control did not receive prior orthodontic treatment either. Patients selected for control were otherwise healthy individuals who presented themselves for treatment of non-skeletal malocclusions, e.g. crowding.
Cephalometric measurement
A total of 73 cephalometric radiographs of patients with and without maxillary micrognathia from one orthodontic clinic were available. A subdivision by gender was not performed. The cephalometric radiographs were measured using the software OnyxCeph® 3TM (Image Instruments GmbH, Chemnitz, Germany).
Landmarks and measuring technique
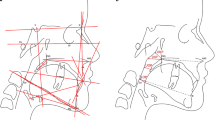
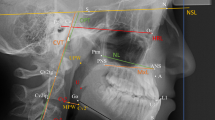
The parameters for evaluation of the cephalometric radiographs were based on landmarks defined and used by Kinzinger et al. [10] and Jonas and Mann [9] for calculating distances and angles (Table 1) in all groups (Fig. 1 and 2).
The angles SNA, SNB, ML-NL, MeGoAr were used to evaluate the sagittal and vertical position of maxilla and mandibula and the growth pattern.
Statistical method, error of the method
Statistical analysis was performed with the SPSS software version 27 (IBM, Armonk, NY, USA). Statistics included Kolmogorov–Smirnov-, T- and Mann–Whitney-U-Tests for the cephalometric radiographs. The level of significance was set at p < 0.05. The significance level was defined as follows: p ≥ 0.05 not significant, p < 0.05 significant, p < 0.01 highly significant and p < 0.001 most highly significant. The effect size was tested by the formula r = Z/√N using Cohen´s criteria (for r): 0.1–0.3 = small effect size and low correlation, 0.3–0.5 = moderat effect size and correlation, > 0.5 = large effect size and high correlation. For testing the intrarater-reliability the evaluation process was repeated on 25 percent of each group three months after the first investigation to evaluate the impact of landmarking errors, which involved removing and replacing the markings. The differences were statistically analyzed using Dahlberg´s error of the method (MF) with the formula MF = √(∑d2/2n), where d is the difference between two measurement results and n is the number of duplicate measurements [3]. The MF for angular and linear measurements in the present study was < 1 for all measurements.
Results
Cephalometric measurements
Bony structures (Table 2)
For maxillary micrognathia patients (MMP) the length of the clivus was smaller than for maxillary eugnathia patients (MEP) (MMP: 35.02 ± 5.01 mm; MEP: 36.55 ± 4.51 mm; p = 0.178). For MMP the posterior upper face height was smaller than for MEP (MMP: 42.19 ± 4.12 mm; MEP: 43.71 ± 2.59 mm; p = 0.601).
Posterior airway space (Table 3)
For MMP the depth of the posterior airway space at the palatal level was significantly smaller than for MEP (MMP: 21.26 ± 3.86 mm; MEP: 23.30 ± 4.17 mm (p = 0.034; r = 0,245)). For MMP the depth of the posterior airway space at the occlusal plane level was bigger than for MEP (MMP: 20.19 ± 4.66 mm; MEP: 20.09 ± 3.47 mm; p = 0.918). For MMP the depth of the posterior airway space at the level of C2 was bigger than for MEP (MMP: 9.71 ± 2.93 mm; MEP: 9.43 ± 3.67 mm; p = 0.724). For MMP the depth of the posterior airway space at the mandibular level was bigger than for MEP (MMP: 10.98 ± 2.98 mm; MEP: 9.70 ± 3.04 mm; p = 0.074). For MMP the depth of the posterior airway space at the level of C3 was bigger than for MEP (MMP: 9.77 ± 4.31 mm; MEP: 9.03 ± 4.12 mm; p = 0.455). For MMP the depth of the posterior airway space at the level of C4 was bigger than for MEP (MMP: 13.55 ± 5.00 mm; MEP: 11.75 ± 3.68 mm; p = 0.149).
Adenoids size and percentages (Table 4)
For MMP the superior area of the adenoids was smaller than for MEP (MMP: 193.49 ± 63.71 mm2; MEP: 196.77 ± 43,90 mm2; p = 0.796). For MMP the entire area of the adenoids was smaller than for MEP (MMP: 317.94 ± 103.45 mm2; MEP: 335.28 ± 76.63 mm2; p = 0.415). For MMP the percentage of the adenoids in relation to the bony nasopharynx was smaller than for MEP (MMP: 68.12 ± 10.60%; MEP: 69.96 ± 11.31%; p = 0.492). For MMP the percentage of the adenoids in relation to the entire nasopharynx was smaller than for MEP (MMP: 56.52 ± 11.68%; MEP: 60.06 ± 12.04%; p = 0.207).
Angles (Table 5)
For MMP the angle ML-NL was bigger than for MEP (MMP: 27.03 ± 5.82°; MEP: 25.70 ± 7.30°; p = 0.397). For MMP the angle MeGoAr was bigger than for MEP (MMP: 127.23 ± 5.63°; MEP: 126.43 ± 6.59°; p = 0.585).
Discussion
Eligibility of the imaging
Adequate dimensions of the airway are prerequisites for normal breathing. Many studies showed that skeletal anomalies are predisposing factors for upper airway obstruction [8]. Cephalometric radiographs have been used widely for evaluation of craniofacial growth. Analyses for dental and skeletal anomalies and the soft tissue have been established. With cephalometric radiographs it is possible to evaluate anomalies in sagittal and vertical direction, but not in the transverse. The resulting limitation has been discussed controversially with regard to the evaluation of the posterior airway space and the adenoids. Some authors recommend other techniques for the evaluation of the upper airway, such as CT scans [7], fluoroscopy [25], fiberoptic pharyngoscopy [19] or magnetic resonance imaging [20]. In dentistry, these techniques are normally not available or indicated. Beyond that cephalometric radiographs are less expensive and carried out with less radiation. Particularly the evaluation of distances and areas are discussed as meaningful parameters to define the airway characteristics. Therefore, cephalometric radiographs are seen as valid diagnostic tools. There are many studies existing using this approach [4, 15, 22, 26]. The accuracy could have been optimized using cone beam computed tomography (CBCT) for three-dimensional analysis. The radiation exposure is less than for computed tomography, but indication setting is strict [13].
Many factors affect the upper airway such as the size of the adenoids, the length and axial area of the airway and the patient being positioned during taking the radiograph. Especially in younger patients, the size of the adenoids changes continuously and seems to be stable at the age of 14 to 15 years. Before that age, adenoids mostly affect the nasopharyngeal volume. The volume of the posterior airway space varies furthermore depending on the respiratory cycle influencing the measurements of the cephalometric radiographs. This problem can be solved by instructing patients to hold their breath during the X-ray. Therefore, cephalometric radiographs are helpful for screening, but for diagnosis of airway obstruction further otolaryngologic diagnostic techniques are required [5].
Since orthodontic appliances tend to affect the morphology of the maxilla, our research was restricted to growing patients without any orthodontic treatment only. At this age, cephalometric radiographs are useful to get an overview of the respiratory airway and the adenoids especially regarding craniofacial growth [11, 14, 23, 27]. Nevertheless, the observational data of the study must be interpreted in association with growth processes. Knowledge of the development of the nasopharyngeal zone is for interpretation mandatory [10]. For adult patients with obstructive airway problems, other otolaryngologic diagnostic tools are needed.
Patients with maxillary prognathia or mandibular retrognathia were included in the study. These sagittal anomalies of the jaws affect the posterior airway space. Nevertheless, they had to be included to the study to build a control group for comperative data generation. Otherwise, the size of the control group would have been too small for valid conclusions.
Growth of nasopharynx and adenoids
The cephalometric radiographs used in this study have been taken during the growth spurt. During this, the tonsilla pharyngea is significantly involved in the depth of the airway. Some studies resulted in different growth pattern of the adenoids not following the growth of the rest of the lymphoid tissues. Increased growth and degeneration appear alternately with two growth peaks at the age of five and nine to twelve years [12]. Preston et al. [18] talk about the maximum of the lymphoid thickness at the age of eleven to twelve years for boys and thirteen to fourteen years for girls. Park et al. [17] proved the correlation of hypo- and hyperdivergent craniofacial growth with regard to different growth pattern and intensity of the degeneration of the tonsilla pharyngea. On these grounds measurements of the depth of the nasopharynx of patients with maxillary micrognathia make sense. Growth of the nasopharynx follows the growth of the body in a constant way. The increase in height is particularly linear and leads back to the subsidence of the hard palate. Scheerer and Lammert [21] incorporated adults in their study. They described an increasing volume of the nasopharynx because of remodeling processes of the maxilla. They also had plaster models of the nasopharynx and gave evidence of its transverse dimension. The width of the nasopharynx did not increase significantly mainly because auf increasing growth of the eustachian tube. These reports confirmed cephalometric radiographs as a useful method for evaluation of the height and depth of the airway. Despite the limitations, cephalometrcic radiographs are valid tools for evaluation of the respiratory airway, especially of the retronasal area and the adenoids.
Conclusion
Patients with maxillary micrognathia showed a bigger extrathoracic airway than the patients with maxillary eugnathia apart from the nasopharynx. Its sagittal dimension tends to be smaller in case of maxillary micrognathia. Bony variations are a probable reason for this because the size of the lymphatic tissues of the nasopharynx was almost indistinguishable. According to the results, maxillary micrognathia was significantly associated with a smaller depth of the posterior airway space at the palatal level compared to patients with maxillary eugnathia. Since obstruction of the upper airway is a predisposing factor for breathing problems, an open upper airway is indispensable for nasal breathing and improves growth of craniofacial structures.
For a final assessment, an interference of hyperdivergent growth pattern and enlarged tonsillae palatinae as well as the different occlusions should be proved.
Data availability
No datasets were generated or analysed during the current study.
References
Andersson L, Brattström V (1991) Cephalometric analysis of permanently snoring patients with and without obstructive sleep apnea syndrome. Int J Oral Maxillofac Surg 20:159–162. https://doi.org/10.1016/s0901-5027(05)80007-4
Ceylan I, Oktay H (1995) A study on the pharyngeal size in different skeletal patterns. Am J Orthod Dentofacial Orthop 108:69–75. https://doi.org/10.1016/s0889-5406(95)70068-4
Dahlberg G (1940) Statistical Methods for Medical and Biological Students. Intersience Publications, New York
de Freitas MR, Alcazar NM, Janson G, de Freitas KM, Henriques JF (2006) Upper and lower pharyngeal airways in subjects with class I and class II malocclusions and different growth patterns. Am J Orthod Dentofacial Orthop 130:742–745. https://doi.org/10.1016/j.ajodo.2005.01.033
Feng X, Li G, Qu Z, Liu L, Näsström K, Shi XQ (2015) Comparative analysis of upper airway volume with lateral cephalograms and cone-beam computed tomography. Am J Orthod Dentofacial Orthop 147:197–204. https://doi.org/10.1016/j.ajodo.2014.10.025
Franke R (2007) Kephalometrische Charakterisierung eines kieferorthopädischen Patientenkollektivs anhand multivariat-statistischer Analysen. Dissertation, Greifswald. https://nbn-resolving.org/urn:nbn:de:gbv:9-000396-3
Haponik EF, Smith PL, Bohlman ME, Allen RP, Goldman SM, Bleecker ER (1983) Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis 127:221–226. https://doi.org/10.1164/arrd.1983.127.2.221
Jena AK, Singh SP, Utreja A (2010) Sagittal mandibular development effects on the dimensions of the awake pharyngeal airway passage. Angle Orthod 80:1061–1067. https://doi.org/10.2319/030210-125.1
Jonas I, Mann W (1988) Zur Bedeutung der Adenoide bei kieferorthopädischen Patienten [The importance of the adenoids in orthodontic patients]. Fortschr Kieferorthop 49:239–251. https://doi.org/10.1007/BF02164447
Kinzinger G, Czapka K, Ludwig B, Glasl B, Gross U, Lisson JA (2011) Effekte festsitzender Apparaturen zur Korrektur der Angle Klasse II auf die Tiefe des extrathorakalen Luftraums. J Orofac Orthop 72:301–320
Lan Y, Chen J, Chen S, He Y, Huang F (2023) Influences of adenoid hypertrophy on children’s maxillofacial development. Healthcare (Basel) 11:2812. https://doi.org/10.3390/healthcare11212812
Linder-Aronson S (1983) The development of the posterior nasopharyngeal wall in boys and girls between the ages of 3–16 years. A longitudinal study. Zahn Mund Kieferheilkd Zentralbl 71:149–160
Ludlow JB, Ivanovic M (2008) Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:106–114. https://doi.org/10.1016/j.tripleo.2008.03.018
Macari AT, Ghafari JG (2023) Secondary analysis of airway obstruction in children with adenoid hypertrophy: association with jaw size and position. Sem Orthod 29:264–270
Martin O, Muelas L, Viñas MJ (2011) Comparative study of nasopharyngeal soft-tissue characteristics in patients with Class III malocclusion. Am J Orthod Dentofacial Orthop 139:242–251. https://doi.org/10.1016/j.ajodo.2009.07.016
McNamara JA (1981) Influence respiratory pattern on craniofacial growth. Angle Orthod 51:269–300. https://doi.org/10.1043/0003-3219(1981)051%3c0269:IORPOC%3e2.0.CO;2
Park JE, Gray S, Bennani H, Antoun JS, Farella M (2016) Morphometric growth changes of the nasopharyngeal space in subjects with different vertical craniofacial features. Am J Orthod Dentofacial Orthop 150:451–458. https://doi.org/10.1016/j.ajodo.2016.02.021
Preston CB (2004) Cephalometric evaluation and measurement of the upper airway. Semin Orthod 10:3–15
Remmers JE, deGroot WJ, Sauerland EK, Anch AM (1978) Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44:931–938. https://doi.org/10.1152/jappl.1978.44.6.931
Rodenstein DO, Dooms G, Thomas Y, Liistro G, Stanescu DC, Culée C, Aubert-Tulkens G (1990) Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax 45:722–727. https://doi.org/10.1136/thx.45.10.722
Scheerer WD, Lammert F (1980) Morphologie und Wachstum des Nasenrachenraumes von 3 Jahren bis zum Erwachsenenalter [Morphology and growth of the nasopharynx from three years to maturity (author’s transl)]. Arch Otorhinolaryngol 229:221–229. https://doi.org/10.1007/BF02565525
Schneider JLGF (2022) Einfluss der maxillären Mikrognathie auf die Morphologie von PAS und Adenoiden. Dissertation, Homburg. https://doi.org/10.22028/D291-40071
Sfondrini MF, Gallo S, Pascadopoli M, Gandini P, Roncoroni C, Scribante A (2024) Upper Airway Dimensions among Different Skeletal Malocclusions: A Retrospective Observational Study by Cephalometric Analysis. Dent J (Basel) 12:12. https://doi.org/10.3390/dj12010012
Sorensen H, Solow B, Greve E (1980) Assessment of the nasopharyngeal airway. A rhinomanometric and radiographic study in children with adenoids. Acta Otolaryngol 227-232. https://doi.org/10.3109/00016488009127132
Suratt PM, Dee P, Atkinson RL, Armstrong P, Wihoit SC (1983) Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis 127:487–492. https://doi.org/10.1164/arrd.1983.127.4.487
Wolford LM, Perez D, Stevao E, Perez E (2012) Airway space changes after nasopharyngeal adenoidectomy in conjunction with Le Fort I osteotomy. J Oral Maxillofac Surg 70:665–671. https://doi.org/10.1016/j.joms.2011.02.069
Zhao T, Yang Z, Ngan P, Luo P, Zhang J, Hua F, He H (2023) Association between adenotonsillar hypertrophy and dentofacial characteristics of children seeking for orthodontic treatment: a cross-sectional study. J Stomatol Oral Maxillofac Surg 125:101751. https://doi.org/10.1016/j.jormas.2023.101751
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Concept: M. T. and J. A. L. Execution and data collection: J. L. F. G. S., M. T. Preparation of Figures and Tables: M. T. Data analysis: J. L. F. G. S., C. C. L, M. T. Manuscript writing: M. T. Revision and approval of manuscript: J. A. L.The final manuscript has been approved by all authors.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Ethical approval for this retrospective study was granted by the Ethical Committee of Ärztekammer des Saarlandes, Saarbrücken, Germany (Decision Number: 117/18).
Informed consent
For this type of study, formal consent is not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tabellion, M., Schneider, J.L.F.G., Linsenmann, C.C. et al. Comparison of PAS and adenoids in patients with and without maxillary micrognathia before orthodontic treatment. Clin Oral Invest 28, 252 (2024). https://doi.org/10.1007/s00784-024-05657-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05657-8