Abstract
Objectives
This study aimed to investigate changes in salivary flow rates, buffering capacity, and salivary chromogranin A (CHGA) levels in adults undergoing bariatric surgery (BS) compared with a non-obese control group.
Materials and methods
Salivary analyses were performed on 62 participants aged over 50 years, stratified into two groups matched for age and gender—individuals who had undergone bariatric surgery (BS) (n = 31) and a corresponding healthy control group (n = 31). Before saliva collection, participants completed a comprehensive 11-point visual numerical rating scale (NRS 0–10) xerostomia questionnaire, assessing subjective perceptions of two key aspects: dryness of the oral mucosa and resultant impact on oral functional ability. Three distinct saliva measurements were obtained: unstimulated whole saliva (UWS), stimulated whole saliva (SWS), and unstimulated upper labial saliva (ULS). The buffering capacity of unstimulated saliva was assessed using pH indicator strips, and concentrations of salivary Chromogranin A (CHGA) were quantified in stimulated saliva via enzyme-linked immunosorbent assay (ELISA).
Results
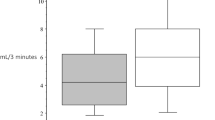
After BS, more than 40% of BS group patients reported xerostomia, with 16.1% experiencing only mild symptoms without significant functional impact (p = 0.009). The prevalence of xerostomia and tongue dryness was higher in the BS group compared to the control group (p = 0.028 and p = 0.025, respectively). The comparative analysis unveiled no statistically significant differences in flow rates of unstimulated upper labial saliva (ULS), unstimulated whole saliva (UWS), and stimulated whole saliva (SWS) between the control group and patients who underwent bariatric surgery. However, in patients undergone BS with xerostomia, both ULS and UWS flow rates were significantly lower than in controls with xerostomia (p = 0.014 and p = 0.007, respectively). The buffering capacity was significantly lower in patients undergone BS than in controls (p = 0.009). No differences were found between groups regarding CHGA concentration and output values, nevertheless, higher values of CHGA concentrations were significantly correlated to lower flow rates.
Conclusion
According to the results, this study suggests that individuals undergoing BS may exhibit altered salivary buffering capacity and reduced unstimulated salivary flows in the presence of xerostomia. Additionally, the findings suggest that elevated concentration of salivary CHGA might be associated, in part, with salivary gland hypofunction.
Clinical relevance
The clinical significance of this study lies in highlighting the changes in salivary functions after BS. The identified salivary alterations might be attributed to adverse effects of BS such as vomiting, gastroesophageal reflux, and dehydration. Understanding these changes is crucial for healthcare professionals involved in the care of post-BS patients, as it sheds light on potential oral health challenges that may arise as a consequence of the surgical intervention. Monitoring and managing these salivary alterations can contribute to comprehensive patient care and enhance the overall postoperative experience for individuals undergoing BS.

Similar content being viewed by others
References
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384:766–781. https://doi.org/10.1016/S0140-6736(14)60460-8
Kopelman PG (2000) Obesity as a medical problem. Nature 404:635–643. https://doi.org/10.1038/35007508
Manohar N, Hayen A, Fahey P, Arora A (2020) Obesity and dental caries in early childhood: a systematic review and meta-analyses. Obes Rev 21:e12960
Jepsen S, Suvan J, Deschner J (2020) The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 2000 83:125–153. https://doi.org/10.1111/prd.12326
Schwenger KJP, Alghamdi MM, Ghorbani Y et al (2020) Hyposalivation is prevalent in bariatric patients but improves after surgery. Surg Obes Relat Dis 16:1407–1413. https://doi.org/10.1016/j.soard.2020.06.005
Modéer T, Blomberg CC, Wondimu B, Julihn A, Marcus C (2010) Association between obesity, flow rate of whole saliva, and dental caries in adolescents. Obesity 18:2367–2373
Grundy SM, Barondess JA, Bellegie NJ et al (1991) Gastrointestinal surgery for severe obesity. Ann Intern Med 115:956–961. https://doi.org/10.7326/0003-4819-115-12-956
Shikora SA, Kim JJ, Tarnoff ME (2007) Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract 22:29–40. https://doi.org/10.1177/011542650702200129
de Carvalho Sales-Peres SH, de Carvalho Sales-Peres M, Ceneviva R, Bernabé E (2017) Weight loss after bariatric surgery and periodontal changes: a 12-month prospective study. Surg Obes Relat Dis 13:637–642. https://doi.org/10.1016/j.soard.2016.08.007
Pataro AL, Cortelli SC, Abreu MHNG et al (2016) Frequency of periodontal pathogens andhelicobacter pylori in the mouths and stomachs of obese individuals submitted to bariatric surgery: a cross-sectional study. J Appl Oral Sci 24:229–238. https://doi.org/10.1590/1678-775720150534
Quintella MCM, Farias TMCP, SoutoMaior JR et al (2020) Relationship between bariatric surgery and dental erosion: a systematic review. Surg Obes Relat Dis 16:1283–1290. https://doi.org/10.1016/j.soard.2020.04.044
Salgado-Peralvo AO, Mateos-Moreno MV, Arriba-Fuente L et al (2018) Bariatric surgery as a risk factor in the development of dental caries: a systematic review. Public Health 155:26–34
Archer-Dubon C, Esquivel-Pedraza L, Ramírez-Anguiano J (2007) Palatal ulcers due to vomiting after gastric band tightening. Obes Surg 17:556–558. https://doi.org/10.1007/s11695-007-9071-9
Hashizume LN, Bastos LF, Cardozo DD et al (2015) Impact of bariatric surgery on the saliva of patients with morbid obesity. Obes Surg 25:1550–1555. https://doi.org/10.1007/s11695-015-1741-4
Knaś M, Maciejczyk M, Sawicka K et al (2016) Impact of morbid obesity and bariatric surgery on antioxidant/oxidant balance of the unstimulated and stimulated human saliva. J Oral Pathol Med 45:455–464. https://doi.org/10.1111/jop.12383
Marsicano JA, Moura-Grec PG, Belarmino LB et al (2011) Interfaces between bariatric surgery and oral health. A longitudinal survey. Acta Cir Bras 26:79–83. https://doi.org/10.1590/s0102-86502011000800015
Bandyopadhyay GK, Mahata SK (2017) Chromogranin A regulation of obesity and peripheral insulin sensitivity. Front Endocrinol (Lausanne) 8:20. https://doi.org/10.3389/fendo.2017.00020
Buchwald H, Estok R, Fahrbach K et al (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122. https://doi.org/10.1016/j.amjmed.2008.09.041
Zhang C, Rigbolt K, Petersen SL et al (2019) The preprohormone expression profile of enteroendocrine cells following Roux-en-Y gastric bypass in rats. Peptides (NY) 118:170100. https://doi.org/10.1016/j.peptides.2019.170100
Kogawa EM, Grisi DC, Falcão DP et al (2016) Salivary function impairment in type 2 Diabetes patients associated with concentration and genetic polymorphisms of chromogranin A. Clin Oral Investig 20:2083–2095. https://doi.org/10.1007/s00784-015-1705-z
Shigeyama C, Ansai T, Awano S et al (2008) Salivary levels of cortisol and chromogranin A in patients with dry mouth compared with age-matched controls. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 106:833–839. https://doi.org/10.1016/j.tripleo.2008.07.005
de Moura-Grec PG, Yamashita JM, Marsicano JA et al (2014) Impact of bariatric surgery on oral health conditions: 6-months cohort study. Int Dent J 64:144–149. https://doi.org/10.1111/idj.12090
Cardozo DD, Hilgert JB, Hashizume LN et al (2014) Impact of bariatric surgery on the oral health of patients with morbid obesity. Obes Surg 24:1812–1816. https://doi.org/10.1007/s11695-014-1364-1
Pai S, Ghezzi EM, Ship JA (2001) Development of a Visual Analogue Scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:311–316. https://doi.org/10.1067/moe.2001.111551
Falcão DP, Leal SC, Vieira CN et al (2014) Sialometry of upper labial minor glands: a clinical approach by the use of weighing method Schirmer’s test strips paper. Sci World J 2014:268634. https://doi.org/10.1155/2014/268634
de Lima DC, Nakata GC, Balducci I, Almeida JD (2008) Oral manifestations of diabetes mellitus in complete denture wearers. J Prosthet Dent 99:60–65. https://doi.org/10.1016/S0022-3913(08)60010-4
Netto BDM, Moreira EAMH, Patiño JSR et al (2012) Influence of Roux-en-Y gastric bypass surgery on vitamin C, myeloperoxidase, and oral clinical manifestations: A 2-year follow-up study. Nutr Clin Pract 27:114–121. https://doi.org/10.1177/0884533611431462
Flink H, Bergdahl M, Tegelberg A, Rosenblad A, Lagerlöf F (2008) Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Commun Dent Oral Epidemiol 36:523–531. https://doi.org/10.1111/j.1600-0528.2008.00432.x
do Nascimento RCRM, Álvares J, Guerra Junior AA et al (2017) Polypharmacy: a challenge for the primary health care of the Brazilian Unified Health System. Rev Saude Publica 51:19s. https://doi.org/10.11606/S1518-8787.2017051007136
Barbe AG (2018) Medication-induced xerostomia and hyposalivation in the elderly: culprits, complications, and management. Drugs Aging 35:877–885. https://doi.org/10.1007/s40266-018-0588-5
Tan ECK, Lexomboon D, Sandborgh-Englund G et al (2018) Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc 66:76–84. https://doi.org/10.1111/jgs.15151
Affoo RH, Foley N, Garrick R et al (2015) Meta-analysis of salivary flow rates in young and older adults. J Am Geriatr Soc 63:2142–2151. https://doi.org/10.1111/jgs.13652
Ivanics T, Nasser H, Leonard-Murali S, Genaw J (2019) Dehydration risk factors and impact after bariatric surgery: an analysis using a national database. Surg Obes Relat Dis 15:2066–2074. https://doi.org/10.1016/j.soard.2019.09.054
Fortes MB, Diment BC, di Felice U, Walsh NP (2012) Dehydration decreases saliva antimicrobial proteins important for mucosal immunity. Appl Physiol Nutr Metab 37:850–859. https://doi.org/10.1139/H2012-054
Syrjänen S (1984) Age-related changes in structure of labial minor salivary glands. Age Ageing 13:159–165. https://doi.org/10.1093/ageing/13.3.159
Vered M, Buchner A, Boldon P, Dayan D (2000) Age-related histomorphometric changes in labial salivary glands with special reference to the Acinar component. Exp Gerontol 35:1075–1084
Eliasson L, Birkhed D, Carlén A (2009) Feeling of dry mouth in relation to whole and minor gland saliva secretion rate. Arch Oral Biol 54:263–267. https://doi.org/10.1016/j.archoralbio.2008.09.001
Roa I, del Sol M (2018) Obesity, salivary glands and oral pathology. Colomb Med 49:280–287. https://doi.org/10.25100/cm.v49i4.3919
Chaudhury NMA, Proctor GB, Karlsson NG et al (2016) Reduced mucin-7 (MUC7) sialylation and altered saliva rheology in Sjögren’s syndrome associated oral dryness. Mol Cell Proteomics 15:1048–1059. https://doi.org/10.1074/mcp.M115.052993
Farooq I, Bugshan A (2020) The role of salivary contents and modern technologies in the remineralization of dental enamel: a narrative review. F1000Res 9:171. https://doi.org/10.12688/f1000research.22499.2
Lamanda A, Cheaib Z, Turgut MD, Lussi A (2007) Protein buffering in model systems and in whole human saliva. PLoS ONE 2:e263. https://doi.org/10.1371/journal.pone.0000263
Battino M, Ferreiro MS, Gallardo I et al (2002) The antioxidant capacity of saliva. J Clin Periodontol 29:189–194. https://doi.org/10.1034/j.1600-051X.2002.290301x.x
Fobi MAL, Lee H, Holness R, Cabinda D (1998) Gastric bypass operation for obesity. World J Surg 22:925–935. https://doi.org/10.1007/s002689900496
Monteforte MJ, Turkelson CM (2000) Bariatric surgery for morbid obesity. Obes Surg 10:391–401. https://doi.org/10.1381/096089200321594246
Korenkov M, Köhler L, Yücel N et al (2002) Esophageal motility and reflux symptoms before and after bariatric surgery. Obes Surg 12:72–76. https://doi.org/10.1381/096089202321144621
Mitchell JE, Lancaster KL, Burgard MA et al (2001) Long-term follow-up of patients’ status after gastric bypass. Obes Surg 11:464–468. https://doi.org/10.1381/096089201321209341
Featherstone JDB (2008) Dental caries: a dynamic disease process. Aust Dent J 53:286–291. https://doi.org/10.1111/j.1834-7819.2008.00064.x
Lussi A, Jaeggi T (2008) Erosion - diagnosis and risk factors. Clin Oral Investig 12:S5-13. https://doi.org/10.1007/s00784-007-0179-z
Castilho AVSS, Foratori-Junior GA, de Carvalho Sales-Peres SH (2019) Bariatric surgery impact on gastroesophageal reflux and dental wear: a systematic review. Arquivos Brasileiros de Cirurgia Digestiva 32:e1466. https://doi.org/10.1590/0102-672020190001e1466
Holbrook WP, Furuholm J, Gudmundsson K et al (2009) Gastric reflux is a significant causative factor of tooth erosion. J Dent Res 5:422–426
Hörchner R, Tuinebreijer W, Kelder H (2002) Eating patterns in morbidly obese patients before and after a gastric restrictive operation. Obes Surg 12:108–112. https://doi.org/10.1381/096089202321144676
Cummings DE, Overduin J, Foster-Schubert KE (2004) Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab 89:2608–2615. https://doi.org/10.1210/jc.2004-0433
Helle KB (2010) Regulatory peptides from chromogranin A and secretogranin II: putative modulators of cells and tissues involved in inflammatory conditions. Regul Pept 165:45–51. https://doi.org/10.1016/j.regpep.2009.09.009
Helle KB, Corti A, Metz-Boutigue MH, Tota B (2007) The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci 64:2863–2886. https://doi.org/10.1007/s00018-007-7254-0
Mahata SK, Corti A (2019) Chromogranin A and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann N Y Acad Sci 1455:34–58. https://doi.org/10.1111/nyas.14249
Acknowledgements
The authors would like to acknowledge Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) for the financial support (grant number 0193.001487/2017) and the present study would not have been possible without the participation of the patients and healthy volunteers.
Funding
This study was funded by Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) (grant number 0193.001487/2017).
Author information
Authors and Affiliations
Contributions
All authors contribute to the study conception and design. Conceptualization and designed the experiments were performed by: E.M.K, F.F.M., L.A.O.B., I.C.R.S., S.H.C.S.P. Material preparation and data collection: E.M.K., F.F.M., R.G.P., L.A.O.B., P.C.C.C., J.L.R. Elisa test: A.P.C.C. Analyzed the data: E.M.K., F.F.M., I.C.R.S., S.H.C.S.P. Writing-original draft preparation: E.M.K, F.F.M. Writing-review and editing: E.M.K, F.F.M., S.H.C.S.P. All authors read, reviewed the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and informed consent
Informed consent was obtained from all individual participants included in the study after a full explanation of the research procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The research protocol was approved by the Ethics Committee of Fundação de Ensino e Pesquisa em Ciências da Saúde/FEPECS/SES/DF (CAAE: 58697816.0.0000.5553, Protocol #1.910.166), Brazil.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kogawa, E.M., Melo, F.F., Pires, R.G. et al. The changes on salivary flow rates, buffering capacity and chromogranin A levels in adults after bariatric surgery. Clin Oral Invest 28, 159 (2024). https://doi.org/10.1007/s00784-024-05551-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05551-3