Abstract
Objectives
This study aimed to assess levels of biomarkers associated with inflammation and tissue destruction in peri-implant crevicular fluid (PICF) of implants provided with customized or standard healing abutments during early implant healing.
Materials and methods
Thirty implants were placed in 22 patients with partial posterior edentulism. Subsequently, test group implants (n=15) received one-piece titanium abutments that were fabricated using computer-aided design/computer-aided manufacturing (CAD/CAM). Control group implants (n=15) were provided with standard abutments. PICF collection and standardized periapical radiographs were carried out at suture removal one week later, following crown delivery after 3 months and at 6 months. Expression of C-reactive protein (CRP), interferon-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12A, IL-17A, macrophage inflammatory protein (MIP)-1α, matrix metalloproteinase (MMP)-13, osteopontin, osteoactivin, Receptor Activator of NF-κB (RANK), and TGF-β were analyzed using a multiplex ELISA kit.
Results
Both groups showed a significant decrease in protein expression of CRP, IL-1β, IL-6, IL-8, MIP-1α, osteopontin, osteoactivin, and TGF-β, while MMP-13 levels increased during the observation period. A rise in OPG and RANK levels was detected among customized abutments. Expression of CRP was higher, whereas IL-1β, IL-1α, and MIP-1α were decreased in control compared to test group implants after 6 months. Marginal bone loss did not depend on abutment modality.
Conclusions
Both abutment types showed distinctive temporal expression of inflammatory biomarkers during 6 months following implant placement.
Trial registration
ISRCTN98477184, registration date 18/05/2022
Clinical relevance
Customized healing abutments exert similar effects on inflammation during early implant healing compared to standard healing abutments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The function and integrity of the peri-implant soft tissue seal play a key role in implant healing by ensuring healthy conditions of the transmucosal area, thereby paving the way for stable osseointegration [1]. The soft tissue seal serves as a protective barrier separating the oral environment from the underlying peri-implant bone and consists of an epithelial and a connective tissue zone [2, 3]. Depending on the prosthetic protocol for non-submerged dental implants, the healing abutment has to be removed and reconnected several times between implant placement and the delivery of the final restoration. As each disconnection of restorative components involves a disruption of the soft tissue adhesion, it has been suggested that repeated manipulations might negatively impact peri-implant soft- and hard-tissue conditions [4,5,6]. In this context, some studies showed adverse effects of the disconnections on marginal bone levels surrounding the implant.
Early investigations by Abrahamsson et al. demonstrated in a pre-clinical study that increasing the number of abutment removals and reconnections up to five times compromised the mucosal barrier, leading to the epithelium’s apical migration and increasing marginal bone resorption [7]. These findings were later confirmed by studies demonstrating that standard prosthetic protocols for implant treatment requiring frequent abutment changes were associated with a decreased subepithelial connective tissue attachment in dogs [5] and with an increased marginal bone loss during the implant healing period in a clinical trial [8]. More specifically, a meta-analysis reported a weighted mean difference in crestal bone loss of 0.19 mm (95% confidence interval, 0.06–0.32 mm) in favor of final abutment placement after implant insertion compared to multiple abutment manipulations [4]. Consequently, it has been suggested that minimizing the number of abutment dis- and reconnections could have beneficial effects on marginal bone levels [9].
Another approach reflecting the tissue conditions surrounding an implant is to analyze the peri-implant crevicular fluid (PICF) [10]. In line with the abovementioned findings on interproximal bone level, repeated abutment removal and reconnection of more than three times were associated with higher IL-1β levels in PICF compared to implants that received final abutments at implant uncovery, suggesting promotion of inflammation [11].
As a result of these studies, prosthetic protocols that aim to reduce tissue trauma following implant insertion have been developed. According to the “one abutment at one time” protocol, the definitive restorative abutment is placed at the time of implant insertion to prevent removal during the healing phase [12]. Moreover, in addition to reducing the number of abutment manipulations, customized abutments can be provided through a virtual design based on computer-guided planning in order to simulate the natural anatomy of the prosthetic crown [13]. To manufacture a customized healing abutment after implant insertion, one digital impression is required which can also be used for the definitive crown design. After the healing period, the abutment is removed in order to attach the crown based on the emergence profile of the abutment. In contrast, conventional impression taking takes at least two abutment manipulations prior to definitive restoration.
However, the influence of minimizing the number of dis- and reconnections of the healing abutment combined with abutment customization on the underlying processes of early implant healing is poorly understood. This pilot study aimed to assess the effects of one-piece customized titanium abutments on a broad range of biomarkers associated with inflammation and tissue degradation in the peri-implant crevicular fluid (PICF) as well as the marginal bone loss observed during the early healing phase. We hypothesized that the use of customized healing abutments induces a reduced inflammatory response compared to standard healing abutments.
Materials and methods
Study population and design
In this prospective study, participants were recruited at the University Clinic of Dentistry, Vienna, between January 2019 and July 2021. Patients were included after obtaining their written consent and if the following inclusion criteria were met: (1) >18 years old, (2) one or more missing tooth/teeth in the molar region of the upper and/or lower jaw, (3) adequate bone quality and availability for implant placement, (4) no signs of inflammation in the region where implant placement is planned, (5) good systemic health conditions, (6) stable occlusion, and (7) willing to participate and attend follow-up appointments. Patients were excluded in case of the following criteria: the presence of untreated periodontitis, smokers (> 10 cigarettes per day), alcoholism or drug abuse, history of chemotherapy or radiation, and diabetes with > 7.5 HbA1c. A total of 30 titanium implants (C1, MIS Implants Technologies, Bar Lev Industrial Park, Israel) were allocated to two groups using online available randomization tools (https://www.randomizer.org/). The general study design is summarized in Fig. 1. The study protocol was approved by the Ethics Committee of the Medical University of Vienna (EK-Nr. 1807/2017) and performed in accordance with the Helsinki Declaration of 1975, as revised in 2013, and the “Good Scientific Practice” guidelines of the Medical University of Vienna. The trial was registered at ISRCTN registry (https://doi.org/10.1186/ISRCTN98477184).
Surgical and prosthetic protocol
Prior to surgery, randomization was performed at each implant site to determine if the implant will be provided with a customized or a standard healing abutment. All patients received 2 g amoxicillin one hour before surgery as a standard clinical protocol [14]. Surgery was performed under local anesthesia using 1–2 cartridges of articaine with 1:100000 epinephrine (Ultracain D-S forte, Sanofi, France) for each implant site. Following a crestal incision and extension to the buccal and lingual aspects of adjacent teeth, a full-thickness flap was elevated, and site preparation was performed according to the manufacturer’s instruction.
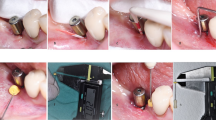
After implant insertion in the test group participants, digital impressions (TRIOS 3, 3shape, Denmark) were taken to manufacture a customized healing abutment from titanium blanks (Ti-blank, MIS Implants Technologies, Bar Lev Industrial Park, Israel) using dental modeling software (Ceramill Mind 3.0, Amann Girrbach, Germany) and a 5-axis milling unit (Ceramill Motion 2, Amann Girrbach, Germany). Customized abutments were subsequently delivered to the patient and installed. In the control group, concave titanium standard healing abutments were installed after surgery. Mucoperiosteal flaps were adapted to the healing abutments and sutured (5-0 coated vicryl, Ethicon, US). Suture removal was performed in all patients one week after implant placement (T1). Three months following surgery, the study participants received a screw-retained crown made out of zirconia (T2). In the control group, conventional impression taking (Impregum Penta Soft Polyether, 3M, US) was performed, and patients received the definitive crown 7 days later. Test group participants were provided with a crown bonded to a titanium base harboring the matching shape of the previous customized healing abutment; the aim was to minimize tissue trauma. Six months after surgery, patients were scheduled for a follow-up visit (T3). Figure 2 shows the installation of a customized abutment followed by crown delivery after 3 months.
Case report for the test and control group. Manufacturing of a CAD/CAM-based customized one-piece titanium abutment (A) and fixation following implant placement (B). Delivery of definitive crown (C) after 3 months (D). Control group implants were provided with standard healing abutments (E) and definitive crown (F)
PICF collection
PICF sample collection was performed on each visit at 4 sites (mesio-buccal, disto-buccal, mesio-lingual, disto-lingual) of each implant. After gentle air drying and isolation of the gingiva with sterile gauze to prevent saliva contamination, a sterilized paper collection strip (PerioPaper strips, Oraflow Inc., Plainview, NY, USA) was inserted into the peri-implant sulcus until slight resistance was reached for 30 s. If contamination with blood was observed, the samples were discarded. The adsorbed volume was determined using a calibrated electronic volume quantification unit (Periotron 8000, Oralflow Inc., Plainview, NY, USA). The four strips of each implant were pooled together in Eppendorf tubes and subsequently stored at −80°C until further processing. Before analysis, the samples were unfrozen, and protein extraction following an elution method was performed as described previously [15]. Twenty microliters of extraction buffer (24.5mL phosphate-buffered saline (pH 7.4), 125ml phenylmethylsulfonylfluoride (PMSF; Sigma Chemical, St. Louis, MO), 200mM in methanol, 1 mg/ml in water, and 83.5 ml of 30% human serum albumin (Sigma Chemical, St. Louis, MO)) were pipetted onto the cellulose part paper collection strip. The strips were put inside Eppendorf tubes and centrifuged at 2000 rpm at 4° C for 5 min. To gain a total volume of 100 μl for each tube, this step was replicated four additional times. The entire product was then stored on dry ice for subsequent analysis of biomarker concentration.
Cytokine analysis
A commercial human multiplex ELISA kit (Quantibody Human Periodontal Disease Array 1 Kit, RayBiotech, Norcross, Georgia, USA) was used to assess the expression of C-reactive protein (CRP), interferon (IFN)-γ, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12A, IL-17A, macrophage inflammatory protein (MIP)-1α, matrix metalloproteinase (MMP)-9, MMP-13, osteopontin, osteoactivin, osteoprotegerin, and Receptor Activator of NF-κB (RANK). Concentrations were determined by generating a standard curve for comparison.
Radiographic assessments
Radiographic examinations using standard parallel technique, perpendicular to the long axis of the implants, were conducted after implant placement (T1) as well as at the 3- and 6-month follow-up visit (T2 and T3, respectively). The distances on the radiograph were calibrated using the known implant diameters. All radiographs were assessed by a blinded independent examiner who was not involved into implant placement or the follow-up appointments (XR). Marginal bone loss (MBL) was determined as the distance from the implant shoulder and the interproximal bone level as described previously [16]. Assessments were performed on the mesial and distal aspect of the implant; they were presented as the mean of the two values for each respective time point.
Statistical analysis
The statistical analysis for all quantitative variables was conducted using SPSS Statistics (IBM, Armonk, NY). The Wilcoxon Signed Rank test was performed to compare the levels of single parameter in PICF samples between different time points. The Mann-Whitney U-test was used to compare the test and control group regarding the levels of cytokine concentration. Quantitative data are expressed and displayed as mean ± SD. P values <0.05 were considered to be statistically significant.
Results
Table 1 shows the demographic and clinical characteristics of the study participants. A total of 30 implants were placed in 22 patients (15 females). Two patients received both test and control group implants. One of these patients was provided with two test and two control group implants. Another patient received two test group implants and one participant was provided with three control group implants. The mean age at the time of implant placement was 47.7 vs. 48.1 years (range 27.1–58.5 vs. 28.9–66.7 years) for the test and control groups, respectively. Five study participants of the test group were smokers (≤10 cigarettes/day), compared to eight in the control group. Implants were most often placed in location 46 (n=6, 40%) in the test group, while location 36 (n=4, 26.7%) was most common in the control group.
Bone level alterations
The results for the marginal bone level (MBL) alterations are presented in Table 2. MBL values significantly decreased in both test and control group at 3-month follow-up (0.24 ± 0.09 mm and 0.23 ± 0.14 mm, respectively, P < 0.01) and at the 6-month follow-up (mean 0.38 ± 0.1mm and 0.33mm ± 0.14 mm, respectively, P<0.01) compared to T1. No significant difference was found between the test and control groups at any milestone.
PICF biomarker changes
The levels in the course of the study of both the test and control groups are presented in Fig. 3. IL-17, IL-12, IL-10, IL-2, IFN-γ, TGF-β, and TNF-α were below the detection limit in the majority of samples, and therefore, these parameters were not included in the analysis. In all samples, MMP-9 was measured above the maximum detection limit; therefore, data were not presented.
The levels of CRP, IL-6, MIP-1α, osteopontin, and osteoactivin gradually decreased at T3 compared to the assessment at suture removal (T1) for the test and control groups. CRP levels of the test group were also lower at T3 than at T2 and decreased in the control group from T1 to T2. IL-6 showed a reduction in the test group from T1 to T2 and from T2 to T3. Also, in the control group, IL-6 levels decreased from T1 to T2. MIP-1α exhibited lower levels at T3 compared to T2 in the test and control groups. IL-8 expression was decreased in the test group at T3 compared to T1. In the control group, IL-1β and TGF-β showed a significantly lower detection level at T3 compared to T1. Osteopontin also decreased in the test group from T1 to T2 and from T2 to T3. Osteoactivin levels diminished in the test and control groups from T1 to T2.
An increase in concentration was observed for IL-1α levels from T2 to T3 in the control group. Also, OPG and RANK levels in the test group were higher at T3 compared to T1. MMP-13 levels increased in the test and control groups from T2 to T3 and in the control group as well from T1 to T3.
No changes in the levels of IFN-γ, IL-2, IL-4, IL-10, IL-12, IL-17, and TNF-α in both groups were observed during the study.
Some differences in the investigated parameters were observed between the test and control groups. At T3, the PICF levels of CRP were superior in the control group than in the test one. Also, IL-1α, IL-1β, and MIP-1α were increased in the test group compared to the control at T3.
Discussion
The results of the present pilot study revealed fading soft tissue inflammation in both treatment groups as well as similar bone remodeling during early implant healing. Some significant intergroup differences in PICF were detected. At 6 months, CRP was less expressed in PICF of the test group than of the control group. CRP is produced in the liver and delivered to the sulcus through saliva and or blood vessels. As blood levels of CRP were not measured in the present study participants, it cannot be ruled out that increased CRP levels could also be due to increased systemic levels in the control group. Furthermore, IL-1β, IL-1α, and MIP-1α were detected at higher levels in the test group compared to the control. IL-1β and IL-1α are pro-inflammatory proteins that are increased in response to growth factors and pro-inflammatory or stress-associated stimuli [17]. In contrast to CRP, production of IL-1β, IL-1α, and MIP-1α takes place locally in the sulcus fluid by macrophages or epithelial cells; this indicates a local enhancement of the inflammation process in PICF of implants provided with customized abutments.
In both abutment groups, a significant decrease in the expression of CRP, IL-6, MIP-1α, osteopontin, and osteoactivin in PICF during the 6-month observation period was measured; this suggests a reduction of the inflammation process. While CRP is known as a marker for systemic inflammation, it has also been detected in PICF at peri-implantitis sites [18]. As a classical pro-inflammatory cytokine, IL-6 has been used to assess peri-implant inflammation in PICF [19]. MIP-1α/CCL3 is secreted by macrophages and plays a role in chemotaxis and stimulation of cell migration during inflammation and bone resorption. It has also been hypothesized to have a potential as a diagnostic tool for peri-implant tissue conditions, although scientific evidence is inconsistent [20, 21]. As a glycosylated phosphoprotein, osteopontin is expressed by both osteoblasts and osteoclasts and is involved in bone resorption and remodeling as well as inflammatory processes [22]. Osteoactivin enhances osteoblast differentiation during matrix maturation and mineralization in osteoblast progenitor cells [23]; however, it has also been associated with inflammation [24].
In contrast to the abovementioned cytokines, both groups showed an increase in the MMP-13 levels in PICF at 6 months compared to suture removal. MMP-13 plays a key role in regulating wound granulation tissue growth and is involved in the expression of genes associated with inflammation, proteolysis, and cell viability [25]. A promotion of MMP-13, therefore, might correlate with wound healing progression during the observation time.
In the test group, an increase in OPG and RANK has been observed after 6 months compared to suture removal following implant placement. OPG and RANK are part of the RANK/RANKL/OPG system, thereby triggering bone metabolism. RANK is an osteoclast-bound receptor, which is activated by its ligand RANKL, resulting in osteoclast differentiation [26]. The present findings suggest an increase in bone turnover in the test group; however, it has to be considered that RANKL was not assessed in the array setting used.
Limited marginal bone loss within the first years following implant placement has been considered an adaptation to surgical trauma and implant loading [27]. No significant differences in the marginal bone levels between the two abutment groups could be identified during the observation period of the present study. Our findings are in line with a study by Moreira et al., who compared the placement of a definitive abutment after implant placement to three times disconnection and reconnection of the healing abutment; they reported a slightly inferior bone loss at 6 months of 0.14 ± 0.18 mm and 0.23 ± 0.29, respectively [28]. However, the present study focused on early implant healing, and possible differences in peri-implant bone level might be detected at a later follow-up visit.
When discussing the present results, it has to be taken into account that other factors in addition to abutment design might also contribute to the difference in cytokine expression between the two abutment groups. In vitro studies have shown that surface material also determines the susceptibility of gingival fibroblasts toward inflammatory stimuli [29]. In line with these findings, the expression of inflammatory cytokines in PICF in clinical settings has been influenced by abutment material [30]. Moreover, also the release of titanium particles during tribocorrosion as a result of material degradation might enhance inflammation. Thus, assessing the specific effects of each abutment modality on the inflammatory process would need further investigation.
This pilot study displays a certain number of limitations. The focus of the present investigation was limited to early implant healing and long-term effects were not assessed. Thus, there is a need for future studies to evaluate the impact of customized one-piece abutments on the healing process over the long-term. Although a broad cytokine profile was assessed in this study, complexity of the inflammation process could only partly be displayed. Also, other aspects essential to the healing process such as angiogenesis, proliferation, or host response could be subject of further studies.
Taken together, customized abutments represent an alternative to standard healing abutments; however, higher production costs and treatment time have to be considered.
Conclusion
Within the limits of this study, we did not observe substantial differences between customized and standard healing abutments with regard to inflammatory markers and marginal bone levels. Subsequently, both treatment protocols can be equally recommended.
Data Availability
Data are available on request from the authors.
References
Sculean A, Gruber R, Bosshardt DD (2014) “Soft tissue wound healing around teeth and dental implants,” (in eng). J Clin Periodontol 41(Suppl 15):S6-22. https://doi.org/10.1111/jcpe.12206
Berglundh T, Lindhe J (1996) Dimension of the periimplant mucosa Biological width revisited. J Clin Periodontol 23(10):971–3. https://doi.org/10.1111/j.1600-051x.1996.tb00520.x. ((in eng))
Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D (1997) “Biologic width around titanium implants A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible,” (in eng). J Periodontol 68(2):186–98. https://doi.org/10.1902/jop.1997.68.2.186
Koutouzis T, Gholami F, Reynolds J, Lundgren T, Kotsakis GA (2017) “Abutment disconnection/reconnection affects peri-implant marginal bone levels: a meta-analysis,” (in eng). Int J Oral Maxillofac Implants 32(3):575–581. https://doi.org/10.11607/jomi.5367
Iglhaut G, Becker K, Golubovic V, Schliephake H, Mihatovic I (2013) “The impact of dis-/reconnection of laser microgrooved and machined implant abutments on soft- and hard-tissue healing,” (in eng). Clin Oral Implants Res 24(4):391–7. https://doi.org/10.1111/clr.12040
Becker K, Mihatovic I, Golubovic V, Schwarz F (2012) “Impact of abutment material and dis-/re-connection on soft and hard tissue changes at implants with platform-switching,” (in eng). J Clin Periodontol 39(8):774–80. https://doi.org/10.1111/j.1600-051X.2012.01911.x
Abrahamsson I, Berglundh T, Lindhe J (1997) “The mucosal barrier following abutment dis/reconnection An experimental study in dogs,” (in eng). J Clin Periodontol 24(8):568–72. https://doi.org/10.1111/j.1600-051x.1997.tb00230.x
Molina A, Sanz-Sánchez I, Martín C, Blanco J, Sanz M (2017) “The effect of one-time abutment placement on interproximal bone levels and peri-implant soft tissues: a prospective randomized clinical trial,” (in eng). Clin Oral Implants Res 28(4):443–452. https://doi.org/10.1111/clr.12818
Perrotti V, Zhang D, Liang A, Wong J, Quaranta A (2019) “The effect of one-abutment at one-time on marginal bone loss around implants placed in healed bone: a systematic review of human studies,” (in eng). Implant Dent 28(6):603–612. https://doi.org/10.1097/ID.0000000000000931
Faot F, Nascimento GG, Bielemann AM, Campão TD, Leite FR, Quirynen M (2015) “Can peri-implant crevicular fluid assist in the diagnosis of peri-implantitis? A systematic review and meta-analysis,” (in eng). J Periodontol 86(5):631–45. https://doi.org/10.1902/jop.2015.140603
Kuppusamy M, Watanabe H, Kasugai S, Kuroda S (2015) “Effects of abutment removal and reconnection on inflammatory cytokine production around dental implants,” (in eng). Implant Dent 24(6):730–4. https://doi.org/10.1097/ID.0000000000000330
Atieh MA, Tawse-Smith A, Alsabeeha NHM, Ma S, Duncan WJ (2017) The one abutment-one time protocol: a systematic review and meta-analysis. J Periodontol 88(11):1173–1185. https://doi.org/10.1902/jop.2017.170238. (in eng)
Long L, Alqarni H, Masri R (2017) “Influence of implant abutment fabrication method on clinical outcomes: a systematic review,” (in eng). Eur J Oral Implantol 10(1):67–77
Laskin DM, Dent CD, Morris HF, Ochi S, Olson JW (2000) “The influence of preoperative antibiotics on success of endosseous implants at 36 months,” (in eng). Ann Periodontol 5(1):166–74. https://doi.org/10.1902/annals.2000.5.1.166
Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC (1995) “Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis A pilot study in beagle dogs,” (in eng). J Clin Periodontol 22(12):903–10. https://doi.org/10.1111/j.1600-051x.1995.tb01793.x
Calvo-Guirado JL et al (2018) “Peri-implant bone loss clinical and radiographic evaluation around rough neck and microthread implants: a 5-year study,” (in eng). Clin Oral Implants Res 29(6):635–643. https://doi.org/10.1111/clr.12775
Di Paolo NC, Shayakhmetov DM (2016) “Interleukin 1α and the inflammatory process,” (in eng). Nat Immunol 17(8):906. https://doi.org/10.1038/ni.3503
Wang IC, Sugai JV, Majzoub J, Johnston J, Giannobile WV, Wang HL (2021) “Pro-inflammatory profiles in cardiovascular disease patients with peri-implantitis,” (in eng). J Periodontol. https://doi.org/10.1002/JPER.21-0419
Hentenaar DFM et al (2021) “Biomarker levels in peri-implant crevicular fluid of healthy implants, untreated and non-surgically treated implants with peri-implantitis,” (in eng). J Clin Periodontol 48(4):590–601. https://doi.org/10.1111/jcpe.13423
Petković AB et al (2010) “Proinflammatory cytokines (IL-1beta and TNF-alpha) and chemokines (IL-8 and MIP-1alpha) as markers of peri-implant tissue condition,” (in eng). Int J Oral Maxillofac Surg 39(5):478–85. https://doi.org/10.1016/j.ijom.2010.01.014
Bhavsar I, Miller CS, Ebersole JL, Dawson DR, Thompson KL, Al-Sabbagh M (2019) “Biological response to peri-implantitis treatment,” (in eng). J Periodontal Res 54(6):720–728. https://doi.org/10.1111/jre.12681
Lund SA, Giachelli CM, Scatena M (2009) “The role of osteopontin in inflammatory processes,” (in eng). J Cell Commun Signal 3(3–4):311–22. https://doi.org/10.1007/s12079-009-0068-0
Abdelmagid SM et al (2008) “Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function,” (in eng). Exp Cell Res 314(13):2334–51. https://doi.org/10.1016/j.yexcr.2008.02.006
Saade M, Araujo de Souza G, Scavone C, Kinoshita PF (2021) “The role of GPNMB in inflammation,” (in eng). Front Immunol 12:674739. https://doi.org/10.3389/fimmu.2021.674739
Toriseva M et al (2012) “MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability,” (in eng). PLoS One 7(8):e42596. https://doi.org/10.1371/journal.pone.0042596
Rakic M, Lekovic V, Nikolic-Jakoba N, Vojvodic D, Petkovic-Curcin A, Sanz M (2013) “Bone loss biomarkers associated with peri-implantitis A cross-sectional study,” (in eng). Clin Oral Implants Res 24(10):1110–6. https://doi.org/10.1111/j.1600-0501.2012.02518.x
Albrektsson T, Chrcanovic B, Östman PO, Sennerby L (2000) 2017 “Initial and long-term crestal bone responses to modern dental implants,” (in eng). Periodontol 73(1):41–50. https://doi.org/10.1111/prd.12176
Moreira F, Rocha S, Caramelo F, Tondela JP (2021) “One-abutment one-time effect on peri-implant marginal bone: a prospective, controlled, randomized, double-blind study,” (in eng). Materials (Basel) 14:15. https://doi.org/10.3390/ma14154179
Andrukhov O et al (2020) “Effect of implant surface material and roughness to the susceptibility of primary gingival fibroblasts to inflammatory stimuli,” (in eng). Dent Mater 36(6):e194–e205. https://doi.org/10.1016/j.dental.2020.04.003
Serichetapongse P, Madarasmi R, Vacharaksa A (2022) “Host responses in peri-implant tissue in comparison to periodontal tissue: a retrospective study,” (in eng). Oral Health Prev Dent 20(1):41–50. https://doi.org/10.3290/j.ohpd.b2585655
Acknowledgements
We thank Mrs. Nguyen Phuong Quynh and Dr. Christoph Kurzmann for excellent technical assistance as well as Dr. Hermann Agis for his scientific expertise.
Funding
Open access funding provided by Medical University of Vienna. This study was funded by the Division of Conservative Dentistry and Periodontology, University Clinic of Dentistry, Medical University of Vienna, Vienna, Austria. This study was also supported by MIS Implants Technologies, Bar Lev Industrial Park, Israel.
Author information
Authors and Affiliations
Contributions
Christian Wehner contributed to study design, patient recruitment, data collection, data interpretation, figure preparation, and drafting and editing the manuscript. Gabor Fürst, Christoph Vasak, and Tom Vaskovich contributed to study concept and clinical realization of the project. Xiaohui Rausch-Fan and Andreas Moritz contributed to study design, data interpretation, and manuscript preparation. Oleh Andrukhov contributed to conceptualization of the project and manuscript, data analysis and interpretation, and statistics and manuscript preparation. All authors approved the final version of manuscript.
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wehner, C., Fürst, G., Vaskovich, T. et al. Effects of customized CAD/CAM abutments on cytokine levels in peri-implant crevicular fluid during early implant healing: a pilot study. Clin Oral Invest 27, 2621–2628 (2023). https://doi.org/10.1007/s00784-022-04826-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04826-x