Abstract
Objective
To compare gingival phenotype assessment methods based on soft tissue transparency on different backgrounds and assessor experience levels.
Methods
For this purpose, 24 gingival specimens were retrieved from pig jaws with tissue thicknesses from 0.2 to 1.25 mm. Three methods were assessed: periodontal probe PCP12 (thin/thick), double-ended periodontal probe DBS12 (thin/moderate/thick) and colour-based phenotype probe CBP (thin/moderate/thick/very thick). Each sample was photographed with each probe underneath and categorized whether the probe was visible or not using different coloured backgrounds. To measure experience level influence, dentists, dental undergraduate students and laypersons (n = 10/group) performed the evaluation.
Results
PCP12 probe showed a threshold between 0.4 and 0.5 mm. To distinct between thin and moderate thick gingiva, a comparable range for DBS12 was found while moderate thickness was between 0.5 and 0.8 mm and for thick above 0.8 mm. CBP also showed a comparable threshold of 0.5 mm for thin versus moderate as compared with the other methods; above 0.8 mm, predominantly a very thick tissue was measured. In general, the background colour had a minor impact on PCP12 and DBS12, and investigator experience showed no clear influence on GP assessment.
Conclusion
Based on probe transparency and within the limitation of a preclinical study, we suggest GP differentiation into three entities: thin (< 0.5 mm; high risk), moderate (0.5–0.8 mm; medium risk) and thick (> 0.8 mm; low risk).
Clinical relevance
All three GP assessment methods are easy to perform and seem to have a high predictive value with a three entities classification for DBS12 and CBP.
Similar content being viewed by others
Introduction
Gingival phenotypes (GP) are assumed to be associated with specific tissue characteristics and dental treatment outcomes. For example, teeth with a thin GP are at greater risk for developing gingival recessions [1] and a thin GP may react more delicately to surgery and heal less predictably when treating gingival recessions. Accordingly, as compared with thicker GPs, more pronounced ridge resorptions can be anticipated after tooth extractions [2]; hence, a thick GP is recommended as one key aspect to reduce the risk for mucosal recession after immediate implant placement [3]. Thick peri-implant tissues seem to be associated with significantly less bone loss [4] and thicker tissues are able to disguise different restorative materials [5].
Eghbali and co-workers [6] showed, however, regardless of clinical experience, the difficulty to visually distinguish between gingival phenotypes. Consequently, different methods were proposed based on the measurement of buccal gingival thickness to correctly assess gingival phenotypes. The simplest non-invasive method was described by Kan et al. [7] based on transparency of a periodontal probe through the gingival margin and has shown the highest predictability an being visible for a gingival thickness (GT) < 0.6 mm (thin GP) and being less or not visible for > 1.0 mm (thick GP). GTs in between were less distinguishable applying a dichotomous classification. Case selection is of utmost importance before immediate implant placement and immediate restoration. Recently, the 5-year outcome of immediate implants has been presented in the aesthetic zone after careful case selection with only thick GP based on the above-mentioned dichotomous classification [8]. Eight out of 17 patients presented with a severe aesthetic complication, e.g. > 1 mm mucosal recession, raising the question whether only thick GP were selected or also patients with an intermediate tissue thickness might have been included partially accounting for the high complication rate.
To overcome the shortcoming of a dichotomous classification, two novel methodologies with corresponding classifications have been developed and introduced. After artificially stratifying a group of young Caucasians into moderately and extremely thin/thick GP, highly statistical significant differences were seen for very thin vs. very thick groups; however, no more differences between the moderate groups were observed [9]. Following these observations, a novel double-ended periodontal probe was evaluated and allowed differentiation into thin (median GT: 0.43 mm), moderate and thick GP (median GT: 0.83 mm). Another GP assessment method based on the visibility of differently coloured probe tips has been presented recently [10]. This approach allowed differentiation between thin, moderate, thick and very thick GP and significant differences in soft tissue response have been observed after orthodontic treatment with more gingival recession and loss of keratinized tissue for thin GP.
To date, no comparative study evaluated different GP classification methods based on probe transparency in a reproducible and standardized fashion. Multiple insertions of different probes within the gingival sulcus might impair the objectivity and reproducibility of the GP assessment by causing swelling or bleeding. In vitro soft tissue transparency measurements have been evaluated already to determine minute colour changes caused by different dental materials assessing soft tissue samples in varying thickness harvested from pig jaws [5, 11]. This approach has been proven to be objective, reproducible and easy to standardize. As another advantage, tissue samples can be harvested in a constant fashion, and camera settings are independent of surrounding influences and photographs can be independently and blindly be assessed by different evaluators.
Therefore, this method has been adopted for the current investigation, in which we aimed to compare different gingival phenotype assessment methods based on soft tissue transparency. Our main objective was to compare three GP assessment methods and to compare their surrogate classifications in relation to direct soft tissue thickness measures. In addition, influence of different tooth shades and investigator experience levels has been evaluated. This study attempted to identify gingival thickness threshold levels corresponding with different GPs.
The hypothesis was that all three methods have specific gingival thickness thresholds and all methods can be applied regardless of assessor experience and/or background colour.
Material and methods
GP assessment tools and classifications
Three different GP assessment methods were used for this ex vivo experiment, each method having a different classification for the determination of GP.
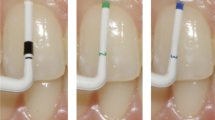
A periodontal probe—exemplary within this study—PCP12 (Hu-Friedy, Chicago, IL, USA) allowing a dichotomous differentiation (thin/thick). Double-ended, colour—as well as tip-thickness-coded periodontal probe DBS12 (Deppeler SA, Rolle, Switzerland) enabling a classification into three categories (thin/moderate/thick). GP-specific tool CBP (Hu-Friedy) with a flat, coloured tip design divides gingival thickness into four subgroups (thin/moderate/thick/very thick).
Sample preparation
Ten freshly slaughtered pork upper jaws were used to retrieve gingival tissue samples. The pigs must have been sacrificed no longer than 2 h before the in vitro tests started. These pigs were raised and slaughtered for food production per local standards for animal welfare. The study protocol did not in any way influence the premortal fate of the animals or the slaughtering process. Therefore, this investigation was not classified as an animal study, and the institutional ethics committee of University of Zurich did not have any objections to the protocol. To avoid possible structural changes in the tissue, the pig jaws were immediately stored in a cooling chamber until the start of the experiment. To avoid structural changes in the tissue, the removed samples were also kept moist in a 0.9% sodium-chloride solution and stored in a cooling chamber until used. With the help of a scalpel, a total of 24 pieces of tissue of different thicknesses were removed from the buccal gingiva of the pig’s jaw. Using a digital calliper (HOLEX, Munich, Germany), the removed tissue pieces were checked for their thickness with an accuracy of 0.01 mm. Sample thickness ranged from 0.2 to 1.25 mm. Sample thickness was measured three times: directly after harvesting, before and after taking pictures with the different probes underneath. All measurements have been in a maximum range of ± 0.02 mm. In order to imitate a clinical situation with different tooth surface colours, three different backgrounds in A2, A3 and A4 were made from polymethyl-methacrylate resin (PMMA; 20 × 20 × 2.5 mm; Enamel Plus Temp, Schütz Dental GmbH, Rosbach, Germany).
To compare the three periodontal probes with each other, the gingival graft samples of different thicknesses were placed one after the other on the different backgrounds. Each probe tip was then inserted between the PMMA background as well as the gingival transplant to a depth of 3 mm and photographed (Fig. 1). With the help of a tripod, which allowed a precise distance between the camera and the gingival transplant of 300 mm comparable with working distance in daily dental practice, standardized pictures could be guaranteed. All pictures were taken with the D7100 SLR camera (Nikon, Minato, Tokyo, Japan) and the EM-140 DG macro flash unit (Sigma, Kawasaki, Kanagawa, Japan).
Four-hundred-thirty-two standardized photographs were taken and inserted in random order into a document from Microsoft PowerPoint 2019 (Microsoft, Redmond, Washington, USA). A total of thirty investigators (ten dentists, ten dental students and ten laypersons) categorized the photos independently of one another in terms of the visibility of the probe through the tissue (visible vs. non-visible).
Statistical evaluation
All statistical analyses and plots were carried out with the statistical software R 4.0.3 including the packages tidyverse, ggplot2 and gplots. An inter-examiner agreement level of > 66.6% was set as a threshold level between two GP subgroups. To test the overall agreement between the different investigators independent of background colour, a uniform assessment threshold level was set to 90% of all investigators.
Results
GP threshold area
The results of all three backgrounds and all examiner groups were considered to assess a transition zone or threshold level for differentiation of gingival phenotype entities (Fig. 2).
Bar graphs showing the results for GP assessment independent of background colour for CBP, DBS12 and PCP12; red lines indicating threshold levels between different GP (CBP: thin/moderate/very thick; DBS12: thin/moderate/thick; PCP12: thin/thick), threshold was set to 66.6% agreement between three investigator groups (lower dotted red line)
Influence of the soft tissue thickness
The soft tissue thickness range for determining the transition from a thin to a thick GP was found to range between 0.4 and 0.5 mm for PCP 12 periodontal probe.
With the help of the double-ended DBS 12, the gingival phenotype could be divided into three groups: thin, moderate and thick. The transition from a thin to a moderate phenotype could be observed with a thickness of the tissue samples also ranging between 0.4 and 0.5 mm, while a moderate to a thick phenotype changed with a thickness between 0.7 and 0.8 mm.
With the colour-coded CBP tips, the transition from a thin to a moderate phenotype was again seen in the range between 0.4 and 0.5 mm. A transition zone from “moderate” to “thick” could not be observed. Instead, a very thick phenotype was seen starting from 0.7 to 0.8 mm.
Influence of the background colour
Evaluating the results for CPB, no tendency or clear pattern was observed between the three background options. To some extent, however, a darker background seemed to shift the decision between thin vs. moderate as well as moderate vs. thick for DBS12 and the moderate GP area was less indifferent possibly due to the higher contrast (Fig. 3). A similar observation was made for PCP12 and differentiation of thin vs. thick GP (A2: 0.5–0.55 mm versus A4: 0.4–0.5 mm).
Influence of the investigator experience
No pattern could be observed, which identified differences based on investigator experience. Hence, further detailed presentation of this data was discarded.
Investigator agreement
For PCP12, an agreement level of ≥ 90% between all participants was seen for soft tissue thicknesses ≤ 0.25 mm (thin GP) and ≥ 0.8 mm (thick GP). For CPB, the 90% level was reached for a thin GP ≤ 0.4 mm, for moderate GP 0.5–0.55 mm and very thick ≥ 1.1 mm; however, no such interval was seen for thick GP. For DBS12, ≥ 90% of all participants choose the same classification for thin GP ≤ 0.4 mm, for thick GP ≥ 0.8 mm and, as partially seen in Fig. 2, agreement for moderate GP is less univocal between 0.5 and 0.8 mm.
Discussion
This study compared three gingival phenotype assessment methods based on transparency through the gingival sulcus and, if possible, aimed to propose a clinically relevant and reproducible classification with corresponding clinical gingival thickness. Furthermore, different investigator groups as well as tooth colour have been chosen as variables. Applying a double-ended periodontal probe (DBS12) or a colour-coded assessment tool (CBP), it seems possible to differentiate between thin (≤ 0.5 mm), moderate and thick (≥ 0.8 mm) gingival phenotypes independent of tooth shade or experience level.
The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions did recommend the use of a traditional periodontal probe based on probe visibility shining through the gingival tissue to differentiate between a thin (≤ 1 mm tissue thickness) and a thick GP (≥ 1 mm) [12]. Differently designed periodontal probes are commercially available with different colour schemes and tip thickness; consequently, different results might be seen clinically and a standardized method might be preferable. Earlier data obtained from a Caucasian population [9, 13, 14] together with this ex vivo report, however, are in contrast with the proposed threshold and do question the application of a simple dichotomous approach. In 2014, GP was evaluated before direct tissue thickness measurement with a pressure-controlled digital calliper—for the first time to the authors knowledge and later used by Liu, Pelekos and Jin [15]—and GP border was around 0.5 mm in young Caucasians [9]. Liu, Pelekos and Jin [15] did not apply a transparency method and set an artificial threshold at 1 mm in a Chinese population. Later, a modified double-ended periodontal probe to allow differentiation into thin/moderate/thick GP [13]. Again, GP was first assessed applying the transparency approach and GT was measured afterwards. Median GT for thin GP was 0.43 mm and for thick GP 0.83 mm supporting our current findings and proposed classification. Another study, however, failed to identify a gingival thickness threshold that could discriminate between thin versus thick GP based on a dichotomous method; nevertheless, a comparable difference in buccal bone and buccal soft tissue thickness was observed [16]. Gingival thickness therefore seems to be directly correlated to the underlying bone thickness and alveolar crest position; hence, pre-surgical evaluation is of high importance before, e.g. immediate implant placement [17].
The International Team of Implantology (ITI) SAC Assessment Tool already differentiates between three GPs to identify high-, medium- and low-risk patients before implant placement and subsequent prosthetic treatment [18]. Trans-gingival probing with either a periodontal probe or an endodontic instrument, ultrasonography or cone beam computer tomography (CBCT) are possible alternatives to the described probe transparency methods; however, these other techniques are either invasive, not available to most clinicians or rather technique sensitive. Probe transparency, in contrast, was recommended as an easy-to-perform, low-cost and non-invasive method for daily clinical practice [19]. Using a simple dichotomous classification as seen for PCP12 (< 0.5 mm >), only high-risk patients with a thin GP could be evaluated. If a more comprehensive approach is warranted, DBS12 or CBP might allow a more advanced differentiation into three subgroups with similar prediction for tissue thickness. Furthermore, distinction between thick and very thick for CBP might not be possible or clinically relevant because of the congruent thickness appraisals for the blue and green tool tip. Supporting this assumption, similar clinical results have been shown for thick and very thick GP based on CBP classification after root coverage procedures without grafts showing; however, no statistically significant differences [20]. Coronally advanced flap procedures yielded complete root coverage in 20% of thin GP, 60% of moderate and ≥ 80% for thick/very thick GP [21]. Future research needs to evaluate treatment protocols based on GP before, e.g. root coverage procedures, implant or orthodontic treatment.
The present study did not evaluate differences of gingival colour, which may be considered as a shortcoming. Potentially, only slight variations within tissue samples could impair the ability to evaluate probe visibility. Tissue samples were strictly kept moist and cooled during the whole investigation; however, slight changes in tissue thickness or colour still might have been occurred. In addition, multiple intraoral measurements at the same location—e.g. six times for our study—might lead to tissue changes as well. While we looked for a high investigator agreement threshold level (≥ 90%) instead of inter-rater reliability or correlation due to inherent statistical calculation and interpretation shortfalls, we did not check intra-rater reliability as another study limitation. In addition, the study assessed non-vital tissues without blood flow which might account for differences to clinical measurements. It remains unclear to which extend gingival pigmentation, collagen content, blood flow and circulation characteristics or surface texture might influence GP assessment. GP differences among ethnicities still need to be evaluated. Furthermore, the influence of different diameter of instrument tips causing different pressure on the gingival margin or within the sulcus cannot be assessed ex vivo. Clinical studies need to confirm the findings of this preclinical investigation.
Conclusion
PCP12, DBS12 and CBP seem to be easy-to-use and reliable tools for daily clinical practice. Based on the above presented preclinical and previous clinical findings of our group, differentiation in more than thin vs. thick GP seems feasible and is advocated. GP classification might be thin (< 0.5 mm, high risk), moderate (0.5–0.8 mm, medium risk) and thick (> 0.8 mm, low risk). Further clinical investigation and clinical trials are needed to approve the above mentioned thresholds and to evaluate the impact on different treatment protocols.
Change history
27 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00784-021-03898-5
References
Cortellini P, Bissada NF (2018) Mucogingival conditions in the natural dentition: narrative review, case definitions, and diagnostic considerations. J Periodontol 89(Suppl 1):S204–S213. https://doi.org/10.1002/JPER.16-0671
Kao RT, Fagan MC, Conte GJ (2008) Thick vs. thin gingival biotypes: a key determinant in treatment planning for dental implants. J Calif Dent Assoc 36:193–198
Cosyn J, Hooghe N, De Bruyn H (2012) A systematic review on the frequency of advanced recession following single immediate implant treatment. J Clin Periodontol 39:582–589. https://doi.org/10.1111/j.1600-051X.2012.01888.x
Di Gianfilippo R, Valente NA, Toti P, Wang H-L, Barone A (2020) Influence of implant mucosal thickness on early bone loss: a systematic review with meta-analysis. J Periodontal Implant Sci 50:209–225
Jung RE, Sailer I, Hammerle CH, Attin T, Schmidlin P (2007) In vitro color changes of soft tissues caused by restorative materials. Int J Periodontics Restorative Dent 27:251–257
Eghbali A, De Rouck T, De Bruyn H, Cosyn J (2009) The gingival biotype assessed by experienced and inexperienced clinicians. J Clin Periodontol 36:958–963. https://doi.org/10.1111/j.1600-051X.2009.01479.x
Kan JY, Rungcharassaeng K, Umezu K, Kois JC (2003) Dimensions of peri-implant mucosa: an evaluation of maxillary anterior single implants in humans. J Periodontol 74:557–562. https://doi.org/10.1902/jop.2003.74.4.557
Cosyn J, Eghbali A, Hermans A, Vervaeke S, De Bruyn H, Cleymaet R (2016) A 5-year prospective study on single immediate implants in the aesthetic zone. J Clin Periodontol 43:702–709. https://doi.org/10.1111/jcpe.12571
Fischer KR, Richter T, Kebschull M, Petersen N, Fickl S (2014) On the relationship between gingival biotypes and gingival thickness in young Caucasians. Clin Oral Implants Res 26:865–869. https://doi.org/10.1111/clr.12356
Rasperini G, Acunzo R, Cannalire P, Farronato G (2015) Influence of periodontal biotype on root surface exposure during orthodontic treatment: a preliminary study. Int J Periodontics Restorative Dent 35:665–675. https://doi.org/10.11607/prd.2239
Jung RE, Holderegger C, Sailer I, Khraisat A, Suter A, Hammerle CH (2008) The effect of all-ceramic and porcelain-fused-to-metal restorations on marginal peri-implant soft tissue color: a randomized controlled clinical trial. Int J Periodontics Restorative Dent 28:357–365
Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, Demirel K, de Sanctis M, Ercoli C, Fan J, Geurs NC, Hughes FJ, Jin L, Kantarci A, Lalla E, Madianos PN, Matthews D, McGuire MK, Mills MP, Preshaw PM, Reynolds MA, Sculean A, Susin C, West NX, Yamazaki K (2018) Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89(Suppl 1):S237–S248. https://doi.org/10.1002/JPER.17-0733
Fischer KR, Kunzlberger A, Donos N, Fickl S, Friedmann A (2018) Gingival biotype revisited-novel classification and assessment tool. Clin Oral Investig 22:443–448. https://doi.org/10.1007/s00784-017-2131-1
Fischer KR, Richter T, Friedmann A, Fickl S (2016) On the relationship between gingival morphotypes and different crown shape assessments in young Caucasians. Clin Oral Investig 20:2185–2190. https://doi.org/10.1007/s00784-016-1720-8
Liu F, Pelekos G, Jin LJ (2017) The gingival biotype in a cohort of Chinese subjects with and without history of periodontal disease. J Periodontal Res 52:1004–1010. https://doi.org/10.1111/jre.12471
Frost NA, Mealey BL, Jones AA, Huynh-Ba G (2015) Periodontal biotype: gingival thickness as it relates to probe visibility and buccal plate thickness. J Periodontol 86:1141–1149. https://doi.org/10.1902/jop.2015.140394
Cook DR, Mealey BL, Verrett RG, Mills MP, Noujeim ME, Lasho DJ, Cronin RJ Jr (2011) Relationship between clinical periodontal biotype and labial plate thickness: an in vivo study. Int J Periodontics Restorative Dent 31:345–354
Dawson A, Chen S, Buser D, Cordaro L, Martin W, Belser U (2009) The SAC classification in implant dentistry. Quintessenz Publishing Co. Ltd., London
Malpartida-Carrillo V, Tinedo-Lopez PL, Guerrero ME, Amaya-Pajares SP, Ozcan M, Rosing CK (2020) Periodontal phenotype: a review of historical and current classifications evaluating different methods and characteristics. J Esthet Restor Dent. https://doi.org/10.1111/jerd.12661
Rasperini G, Codari M, Limiroli E, Acunzo R, Tavelli L, Levickiene AZ (2019) Graftless tunnel technique for the treatment of multiple gingival recessions in sites with thick or very thick biotype: a prospective case series. Int J Periodontics Restorative Dent 39:e203–e210. https://doi.org/10.11607/prd.4134
Rasperini G, Codari M, Paroni L, Aslan S, Limiroli E, Solis-Moreno C, Suckiel-Papior K, Tavelli L, Acunzo R (2020) The influence of gingival phenotype on the outcomes of coronally advanced flap: a prospective multicenter study. Int J Periodontics Restorative Dent 40:e27–e34. https://doi.org/10.11607/prd.4272
Acknowledgements
The authors deeply appreciate the help of Mrs. Andrea Gubler for assistance in sample processing and of Dr. Daniel Wiedemeier for his advice during statistical evaluation and plot design.
Funding
Open Access funding provided by Universität Zürich. The work was supported by the Clinic of Conservative & Preventive Dentistry, Division of Periodontology & Peri-implant Diseases, Centre of Dental Medicine, University of Zurich, Zurich, Switzerland
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to conception and design of the study. KF & JB have been involved in data collection, data analysis and data interpretation. KF & PS have drafted the first versions of the manuscript, while TT, GR & TA have revised it critically and have given final approval of the version to be published.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies involving human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
KF, JB, TA & PA declare that they have no conflict of interest. TT & GR did not participate in data collection and declare a financial interest with the phenotype probe produced by Hu-Friedy.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was originally published without an incomplete affiliation. The complete affiliation is: Clinic of Conservative and Preventive Dentistry, Division of Periodontology and Peri-implant Diseases Centre of Dental Medicine, University of Zurich, Zurich, Switzerland.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, K.R., Büchel, J., Testori, T. et al. Gingival phenotype assessment methods and classifications revisited: a preclinical study. Clin Oral Invest 25, 5513–5518 (2021). https://doi.org/10.1007/s00784-021-03860-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-03860-5