Abstract
Objectives
The current study aimed to investigate the effects of vascular endothelial growth factor (VEGF) and stromal cell-derived factor-1α (SDF-1α) overexpressing dental pulp stem cells (DPSCs) in vascularized dental pulp regeneration in vivo.
Materials and methods
Human DPSCs were transfected with VEGF or SDF-1α using premade lentiviral particles. Overexpression was verified by quantitative polymerase chain reaction (q-PCR), enzyme-linked immunosorbent assay (ELISA), and western blot analysis. Effects of SDF-1α and VEGF overexpressing DPSCs on their proliferation (CCK-8 and MTT assays) and endothelial vascular-tube formation (Matrigel assay) were investigated in vitro. Human tooth roots sectioned into 6-mm segments were injected with gene-modified DPSCs encapsulated in PuraMatrix hydrogel and implanted in the dorsum of severe-combined-immunodeficient (SCID) mice. Implants were retrieved after 4 weeks and examined for regenerated pulp-like tissue and vascularization using histology and immunohistochemistry. p < 0.05 was considered statistically significant.
Results
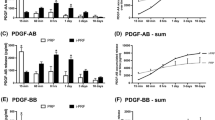
Gene-modified DPSCs expressed significantly high levels (p < 0.05) of SDF-1α and VEGF mRNA and proteins, respectively. Transfected DPSCs showed a significantly higher cell proliferation compared to that of wild-type DPSCs. Furthermore, they enhanced endothelial cell migration and vascular-tube formation on Matrigel in vitro. When injected into tooth root canals and implanted in vivo, DPSCs/SDF-1α + DPSCs/VEGF-mixed group resulted in significantly increased length of regenerated pulp-like tissue within the root canals compared to that of wild-type DPSCs/VEGF and DPSCs/SDF-1α groups. Vessel area density was significantly higher in DPSCs/SDF-1α and mixed DPSCs/SDF-1α + DPSCs/VEGF groups than in DPSCs-VEGF alone or wild-type DPSCs groups.
Conclusion
A combination of VEGF-overexpressing and SDF-1α-overexpressing DPSCs could enhance the area of vascularized dental pulp regeneration in vivo.
Clinical relevance
Enhancing vascularization in pulp regeneration is crucial to overcome the clinical limitation of the limited blood supply to the root canals via a small apical foramen enclosed by hard dentin.








Similar content being viewed by others
References
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2010) Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng A 16:605–615. https://doi.org/10.1089/ten.TEA.2009.0518
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535
Batouli S, Miura M, Brahim J, Tsutsui T, Fisher L, Gronthos S, Robey PG, Shi S (2003) Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 82:976–981
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630. https://doi.org/10.1073/pnas.240309797
Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M (2006) Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood 107:3546–3554
Carmeliet P (2000) VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med 6:1102–1103. https://doi.org/10.1038/80430
Marsboom G, Pokreisz P, Gheysens O, Vermeersch P, Gillijns H, Pellens M, Liu X, Collen D, Janssens S (2008) Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 26:1017–1026
Das R, Jahr H, van Osch GJ, Farrell E (2009) The role of hypoxia in bone marrow–derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng B Rev 16:159–168
Grunewald M, Avraham I, Dor Y, Bachar Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124:175–189. https://doi.org/10.1016/j.cell.2005.10.036
Yang J, Zhou W, Zheng W, Ma Y, Lin L, Tang T, Liu J, Yu J, Zhou X, Hu J (2006) Effects of myocardial transplantation of marrow mesenchymal stem cells transfected with vascular endothelial growth factor for the improvement of heart function and angiogenesis after myocardial infarction. Cardiology 107:17–29
Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195–1201
Tang J, Wang J, Zheng F, Kong X, Guo L, Yang J, Zhang L, Huang Y (2010) Combination of chemokine and angiogenic factor genes and mesenchymal stem cells could enhance angiogenesis and improve cardiac function after acute myocardial infarction in rats. Mol Cell Biochem 339:107–118
Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ (2010) Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng A 16:3023–3031
Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C (2015) The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng A 21:550–563. https://doi.org/10.1089/ten.TEA.2014.0154
Dissanayaka WL, Zhu L, Hargreaves KM, Jin L, Zhang C (2014) Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J Dent Res 93:1296–1303. https://doi.org/10.1177/0022034514550040
Zhou W, He DQ, Liu JY, Feng Y, Zhang XY, Hua CG, Tang XF (2015) Angiogenic gene-modified myoblasts promote vascularization during repair of skeletal muscle defects. J Tissue Eng Regen Med 9:1404–1416. https://doi.org/10.1002/term.1692
Zhang W, Liu W, Ling J, Lin Z, Gao Y, Mao X, Jian Y (2014) Odontogenic differentiation of vascular endothelial growth factor-transfected human dental pulp stem cells in vitro. Mol Med Rep 10:1899–1906. https://doi.org/10.3892/mmr.2014.2481
Dissanayaka WL, Zhang C (2017) The role of vasculature engineering in dental pulp regeneration. J Endod 43:S102–S106. https://doi.org/10.1016/j.joen.2017.09.003
Fierro FA, Kalomoiris S, Sondergaard CS, Nolta JA (2011) Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 29:1727–1737. https://doi.org/10.1002/stem.720
Heng BC, Ye X, Liu Y, Dissanayaka WL, Cheung GS, Zhang C (2016) Effects of recombinant overexpression of Bcl2 on the proliferation, apoptosis, and osteogenic/odontogenic differentiation potential of dental pulp stem cells. J Endod 42:575–583. https://doi.org/10.1016/j.joen.2016.01.013
Miller AD, Miller DG, Garcia JV, Lynch CM (1993) Use of retroviral vectors for gene transfer and expression. Methods Enzymol 217:581–599
Vigna E, Naldini L (2000) Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med 2:308–316. https://doi.org/10.1002/1521-2254(200009/10)2:5<308::AID-JGM131>3.0.CO;2-3
Bjorklund A, Bjorklund T, Kirik D (2009) Gene therapy for dopamine replacement in Parkinson’s disease. Sci Transl Med 1:2ps2. https://doi.org/10.1126/scitranslmed.3000350
Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugieres P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA (2014) Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet 383:1138–1146. https://doi.org/10.1016/S0140-6736(13)61939-X
Perna SK, Savoldo B, Dotti G (2014) Genetic modification of cytotoxic T lymphocytes to express cytokine receptors. Methods Mol Biol 1139:189–200. https://doi.org/10.1007/978-1-4939-0345-0_17
Arnaoutova I, Kleinman HK (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5:628–635. https://doi.org/10.1038/nprot.2010.6
Kaigler D, Krebsbach PH, Polverini PJ, Mooney DJ (2003) Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng 9:95–103. https://doi.org/10.1089/107632703762687573
Dissanayaka WL, Zhan X, Zhang C, Hargreaves KM, Jin L, Tong EH (2012) Coculture of dental pulp stem cells with endothelial cells enhances osteo−/odontogenic and angiogenic potential in vitro. J Endod 38:454–463. https://doi.org/10.1016/j.joen.2011.12.024
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864
Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410:50–56
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962–969
Funding
The work was supported by the General Research Fund grants (project codes 17126914 and HKU 784912) awarded to Chengfei Zhang by the Research Grants Council of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants by any of the authors. All applicable institutional guidelines set by the Committee on the Use of Live Animals in Teaching and Research (CULATR) of The University of Hong Kong for the care and use of animals were followed.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Zhu, L., Dissanayaka, W.L. & Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin Oral Invest 23, 2497–2509 (2019). https://doi.org/10.1007/s00784-018-2699-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2699-0