Abstract
Organometallic η6-arene ruthenium(II) complexes with 3-chloro-6-(1H-pyrazol-1-yl)pyridazine (Ru1, Ru2, and Ru5) and 3-chloro-6-(3,5-dimethyl-1H-pyrazol-1-yl)pyridazine (Ru3-4) N,N’ heterocyclic and η6-arene (cymene (Ru1-4) or toluene (Ru 5)) have been synthesized. The ruthenium(II) complexes have common “three-legged piano-stool” pseudo-octahedral structures known for half-sandwich complexes. Evolution of their UV–Visible absorption spectra in PBS buffer or DMSO over 24 h confirmed their good solvolysis stability. Titrations of the complexes with the calf thymus DNA (CT-DNA) were monitored using UV–Visible absorption and fluorescence spectroscopies. The complexes interact moderately with CT-DNA and their binding constants are in the order of 104 M−1. Competitive binding of the complexes to a DNA-Hoechst 33,258 depicted competitive displacement of Hoechst from DNA’s minor grooves. These complexes bind to glutathione forming GSH-adducts through S coordination by replacement of a halide, with the iodo-analogues having higher binding constants than the chloro-complexes. Cyclic voltammograms of the complexes exhibited one electron-transfer quasi-reversible process. Trends in the molecular docking data of Ru1-5/DNA were similar to those for DNA binding constants. Of the five, only Ru1, Ru3 and Ru5 showed some activity (moderate) against the MCF-7 breast cancer cells with IC50 values in the range of 59.2–39.9 for which Ru5 was the most active. However, the more difficult-to-treat cell line, MDA-MB 231 cell was recalcitrant to the treatment by these complexes.
Graphical abstract
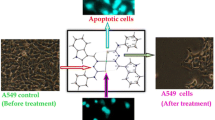
Molecular docking simulations visualized the interactions of arene Ru(II) complexes with CT-DNA via minor grooving. The trends were corroborated by electrochemical and cytotoxicity data.








Similar content being viewed by others
Data availability
The data is available electronically as supplementary information.
References
Bennett JE, Stevens GA, Mathers CD, Bonita R et al (2018) NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 392(10152):1072–1088
Ferlay, J., M. Ervik, F. Lam, M. Colombet, et al., Global cancer observatory: cancer today. Int. Agency Res. International Journal of Cancer, 2020: p. 1–12.
Hartinger CG, Metzler-Nolte N, Dyson PJ (2012) Challenges and opportunities in the development of organometallic anticancer drugs. Organometallics 31(16):5677–5685
Reedijk J (1996) Improved understanding in platinum antitumour chemistry. Chem Commun 7:801–806
Sze JH, Raninga PV, Nakamura K, Casey M et al (2020) Anticancer activity of a Gold (I) phosphine thioredoxin reductase inhibitor in multiple myeloma. Redox Biol 28:101310–110321
Nahari G, Tshuva EY (2021) Synthesis of asymmetrical diaminobis (alkoxo)-bisphenol compounds and their C 1-symmetrical mono-ligated titanium(IV) complexes as highly stable highly active antitumor compounds. Dalton Trans 50(19):6423–6426
Nabiyeva T, Marschner C, Blom B (2020) Synthesis, structure and anti-cancer activity of osmium complexes bearing π-bound arene substituents and phosphane Co-Ligands: A review. Eur J Med Chem 201:112483–112498
Wani WA, Baig U, Shreaz S, Shiekh RA et al (2016) Recent advances in iron complexes as potential anticancer agents. New J Chem 40(2):1063–1090
Allardyce CS, Dyson PJ (2016) Metal-based drugs that break the rules. Dalton Trans 45(8):3201–3209
Mondal A, Paira P (2020) Hypoxia efficient and glutathione-resistant cytoselective ruthenium(II)-p-cymene-arylimidazophenanthroline complexes: biomolecular interaction and live cell imaging. Dalton Trans 49(36):12865–12878
Khanvilkar P, Pulipaka R, Shirsath K, Devkar R et al (2019) Organometallic binuclear Ru(II) complexes: design, synthesis, DNA/BSA binding interactions and in-vitro cytotoxicity against HeLa cell line. Inorg Chem Commun 102:134–140
Gichumbi JM, Friedrich HB (2018) Half-sandwich complexes of platinum group metals (Ir, Rh, Ru and Os) and some recent biological and catalytic applications. J Organomet Chem 866:123–143
Colina-Vegas L, Oliveira KM, Cunha BN, Cominetti MR et al (2018) Anti-proliferative and anti-migration activity of Arene–ruthenium (II) complexes with azole therapeutic agents. Inorganics 6(4):132
Yan YK, Melchart M, Habtemariam A, Sadler PJ (2005) Organometallic chemistry, biology and medicine: ruthenium arene anticancer complexes. Chem Commun 38:4764–4776
Thangavel S, Rajamanikandan R, Friedrich HB, Ilanchelian M et al (2016) Binding interaction, conformational change, and molecular docking study of N-(pyridin-2-ylmethylene) aniline derivatives and carbazole Ru(II) complexes with human serum albumins. Polyhedron 107:124–135
Habtemariam A, Melchart M, Fernández R, Parsons S et al (2006) Structure-activity relationships for cytotoxic ruthenium (II) arene complexes containing N, N-, N, O-, and O O-chelating ligands. J Med Chem 49(23):6858–6868
Hanif M, Meier SM, Nazarov AA, Risse J et al (2013) Influence of the π-coordinated arene on the anticancer activity of ruthenium(II)carbohydrate organometallic complexes. Front Chem 1:27
Mendoza-Ferri MG, Hartinger CG, Nazarov AA, Eichinger RE et al (2009) Influence of the arene ligand, the number and type of metal centers, and the leaving group on the in vitro antitumor activity of polynuclear organometallic compounds. Organometallics 28(21):6260–6265
Morris RE, Aird RE, del Socorro Murdoch P, Chen H et al (2001) Inhibition of cancer cell growth by ruthenium(II) arene complexes. J Med Chem 44(22):3616–3621
Elguero, J., Comprehensive Heterocyclic Chemistry II. Journal: Comprehensive Heterocyclic Chemistry II, 1996: p. 1–75.
Dogné J-M, Supuran CT, Pratico D (2005) Adverse cardiovascular effects of the coxibs. J Med Chem 48(7):2251–2257
Padma-Nathan H (2006) Sildenafil citrate (Viagra) treatment for erectile dysfunction: an updated profile of response and effectiveness. Int J Impot Res 18(5):423–431
Mizuhara T, Kato T, Hirai A, Kurihara H et al (2013) Structure–activity relationship study of phenylpyrazole derivatives as a novel class of anti-HIV agents. Bioorg Med Chem Lett 23(16):4557–4561
Pal D, Saha S, Singh S (2012) Importance of pyrazole moiety in the field of cancer. J Pharm Pharm Sci 4(2):98–104
Gichumbi JM, Friedrich HB, Omondi B (2016) Synthesis and characterization of half-sandwich ruthenium(II) complexes with N-alkyl pyridyl-imine ligands and their application in transfer hydrogenation of ketones. Transition Met Chem 41(8):867–877
Mambanda A, Ongoma P, Gichumbi J, Omondi RO et al (2022) Crystal structures of half-sandwich Ru(II) complexes,[(η6-p-Cymene)(3-chloro-6-(1 H-pyrazol-1-yl) pyridazine) Ru (X)] BF4,(X= Cl, Br, I). Molbank 2022(4):M1477
Prasad KT, Therrien B, Rao KM (2008) Cationic half-sandwich complexes (Rh, Ir, Ru) containing 2-substituted-1, 8-naphthyridine chelating ligands: syntheses, X-ray structure analyses and spectroscopic studies. J Organomet Chem 693(18):3049–3056
Monro S, Colon KL, Yin H, Roque J III et al (2018) Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem Rev 119(2):797–828
Romero-Canelón I, Salassa L, Sadler PJ (2013) The contrasting activity of iodido versus chlorido ruthenium and osmium arene azo-and imino-pyridine anticancer complexes: control of cell selectivity, cross-resistance, p53 dependence, and apoptosis pathway. J Med Chem 56(3):1291–1300
Gupta G, Prasad KT, Das B, Yap GP et al (2009) Ruthenium half-sandwich complexes with tautomerized pyrazolyl-pyridazine ligands: synthesis, spectroscopic and molecular structural studies. J Organomet Chem 694(16):2618–2627
Gupta G, Prasad KT, Rao AV, Geib SJ et al (2010) Novel mononuclear η5-pentamethylcyclopentadienyl complexes of platinum group metals bearing pyrazolylpyridazine ligands: syntheses and spectral studies. Inorg Chim Acta 363(10):2287–2295
Gichumbi JM, Friedrich HB, Omondi B (2016) Solvato-polymorph of [(η6-C6H6)RuCl (L)]PF6 (L=(2, 6-dimethyl-phenyl-pyridin-2-yl methylene amine). J Mol Struct 1113:55–59
Patra M, Joshi T, Pierroz V, Ingram K et al (2013) DMSO-mediated ligand dissociation: renaissance for biological activity of N-Heterocyclic-[Ru (η6-arene) Cl2] drug candidates. Chemistry A Europ J 19(44):14768–14772
Moon S, Hanif M, Kubanik M, Holtkamp H et al (2015) Organoruthenium and osmium anticancer complexes bearing a maleimide functional group: reactivity to cysteine, stability, and cytotoxicity. ChemPlusChem 80(1):231–236
Tan C, Liu J, Li H, Zheng W et al (2008) Differences in structure, physiological stability, electrochemistry, cytotoxicity, DNA and protein binding properties between two Ru(III) complexes. J Inorg Biochem 102(2):347–358
Bhattacharyya S, Purkait K, Mukherjee A (2017) Ruthenium (II) p-cymene complexes of a benzimidazole-based ligand capable of VEGFR2 inhibition: hydrolysis, reactivity and cytotoxicity studies. Dalton Trans 46(26):8539–8554
Chakraborty A, Roy S, Chakraborty MP, Roy SS et al (2021) Cytotoxic ruthenium (II) complexes of pyrazolylbenzimidazole ligands that inhibit VEGFR2 phosphorylation. Inorg Chem 60(23):18379–18394
Khanvilkar P, Dash SR, Banerjee D, Vohra A et al (2021) Organoruthenium (II) complexes featuring pyrazole-linked thiosemicarbazone ligands: synthesis, DNA/BSA interactions, molecular docking, and cytotoxicity studies. Appl Organomet Chem 35(10):e6343
Galindo-Murillo R, García-Ramos JC, Ruiz-Azuara L, Cheatham TE et al (2015) Intercalation processes of copper complexes in DNA. Nucleic Acids Res 43(11):5364–5376
Snyder RD, Hendry LB (2005) Toward a greater appreciation of noncovalent chemical/DNA interactions: application of biological and computational approaches. Environ Mol Mutagen 45(2–3):100–105
Pages BJ, Ang DL, Wright EP, Aldrich-Wright JR (2015) Metal complex interactions with DNA. Dalton Trans 44(8):3505–3526
Jaumot J, Gargallo R (2012) Experimental methods for studying the interactions between G-quadruplex structures and ligands. Curr Pharm Des 18(14):1900–1916
Bhadra K, Kumar GS (2011) Interaction of berberine, palmatine, coralyne, and sanguinarine to quadruplex DNA: a comparative spectroscopic and calorimetric study. Biochim Biophys Acta (BBA) Gen Subj 1810(4):485–496
Mukherjee S, Mitra I, Fouzder C, Mukherjee S et al (2017) Effect of Pt(II) complexes on cancer and normal cells compared to clinically used anticancer drugs: cell cycle analysis, apoptosis and DNA/BSA binding study. J Mol Liq 247:126–140
Paitandi RP, Singh RS, Mukhopadhyay S, Sharma G et al (2017) Synthesis, characterization, DNA binding and cytotoxicity of fluoro-dipyrrin based arene ruthenium(II) complexes. Inorg Chim Acta 454:117–127
Paitandi RP, Sharma V, Singh VD, Dwivedi BK et al (2018) Pyrazole appended quinoline-BODIPY based arene ruthenium complexes: their anticancer activity and potential applications in cellular imaging. Dalton Trans 47(48):17500–17514
Čanović P, Simović AR, Radisavljević S, Bratsos I et al (2017) Impact of aromaticity on anticancer activity of polypyridyl ruthenium(II) complexes: synthesis, structure, DNA/protein binding, lipophilicity and anticancer activity. J Biol Inorg Chem 22(7):1007–1028
Alsaeedi MS, Babgi BA, Abdellattif MH, Jedidi A et al (2020) DNA-binding capabilities and anticancer activities of ruthenium(II) cymene complexes with (Poly) cyclic aromatic diamine ligands. Molecules 26(1):76
Purkait K, Karmakar S, Bhattacharyya S, Chatterjee S et al (2015) A hypoxia efficient imidazole-based Ru(II) arene anticancer agent resistant to deactivation by glutathione. Dalton Trans 44(13):5969–5973
Medjedović M, Simović AR, Ćoćić D, Milutinović M et al (2020) Dinuclear ruthenium(II) polypyridyl complexes: mechanistic study with biomolecules DNA/BSA interactions and cytotoxic activity. Polyhedron 178:114334
Maikoo S, Chakraborty A, Vukea N, Dingle LMK et al (2021) Ruthenium complexes with mono-or bis-heterocyclic chelates: DNA/BSA binding, antioxidant and anticancer studies. J Biomol Struct Dyn 39(11):4077–4088
Sankareswari VG, Vinod D, Mahalakshmi A, Alamelu M et al (2014) Interaction of oxovanadium(IV)–salphen complexes with bovine serum albumin and their cytotoxicity against cancer. Dalton Trans 43(8):3260–3272
Balendiran GK, Dabur R, Fraser D (2004) The role of glutathione in cancer. Cell Biochem Funct Cell Biochem Modulat Act Agents Dis 22(6):343–352
Wang F, Chen H, Parkinson JA, Murdoch PDS et al (2002) Reactions of a ruthenium(II) arene antitumor complex with cysteine and methionine. Inorg Chem 41(17):4509–4523
Dougan SJ, Habtemariam A, McHale SE, Parsons S et al (2008) Catalytic organometallic anticancer complexes. Proc Natl Acad Sci 105(33):11628–11633
Matsinha LC, Malatji P, Hutton AT, Venter GA et al (2013) Water-soluble half-sandwich RuII–arene complexes: synthesis, structure, electrochemistry, dft studies, and aqueous phase hydroformylation of 1-octene. Eur J Inorg Chem 2013(24):4318–4328
Wang H, Sayed SY, Luber EJ, Olsen BC et al (2020) Redox flow batteries: how to determine electrochemical kinetic parameters. ACS Nano 14(3):2575–2584
Wu X, Ye R, Jia A-Q, Chen Q et al (2013) Syntheses, crystal structures and electrochemical properties of acetylacetonato-ruthenium complexes containing substituted pyridine ligands. Zeitsch Naturforschung B 68(9):993–999
Pastuszko A, Niewinna K, Czyz M, Jóźwiak A et al (2013) Synthesis, X-ray structure, electrochemical properties and cytotoxic effects of new arene ruthenium(II) complexes. J Organomet Chem 745:64–70
Welsh A, Rylands L-I, Arion VB, Prince S et al (2020) Synthesis and antiproliferative activity of benzimidazole-based, trinuclear neutral cyclometallated and cationic, N^N-chelated ruthenium(II) complexes. Dalton Trans 49(4):1143–1156
Mizumura Y, Matsumura Y, Hamaguchi T, Nishiyama N et al (2001) Cisplatin-incorporated polymeric micelles eliminate nephrotoxicity, while maintaining antitumor activity. Jpn J Cancer Res 92(3):328–336
Gichumbi JM, Omondi B, Lazarus G, Singh M et al (2017) Influence of halogen substitution in the ligand sphere on the antitumor and antibacterial activity of half-sandwich ruthenium(II) complexes [RuX (η6-arene)(C5H4N2-CH= N-Ar)]+. Z Anorg Allg Chem 643(11):699–711
Acknowledgements
We acknowledge the Department of Chemistry at Egerton University in Kenya, where the conception and synthesis of the complexes were carried out. Part of this work (characterization and cell viability studies) was partially funded by the School of Chemistry University of KwaZulu-Natal (UKZN) and the Department of Chemistry, Department of Human Biology, University of Cape Town, RSA, respectively. We thank Mrs. C. J. van Rensburg for assistance in mass spectroscopic analysis and Mr. C. Grimmer for NMR analysis.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No potential conflict of interests or competing interests is foreseen.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanyora, A.K., Omondi, R.O., Ongoma, P. et al. Mononuclear η6-arene ruthenium(II) complexes with pyrazolyl–pyridazine ligands: synthesis, CT-DNA binding, reactivity towards glutathione, and cytotoxicity. J Biol Inorg Chem (2024). https://doi.org/10.1007/s00775-024-02043-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00775-024-02043-3