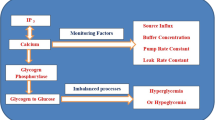
Abstract
In the present work, the multiple-indicator dilution (MID) technique was used to investigate the kinetic mechanisms by which nickel (Ni2+) affects the calcium (Ca2+) transport in intact rat liver. 45Ca2+ and extra- and intracellular space indicators were injected in livers perfused with 1 mM Ni2+, and the outflow profiles were analyzed by a mathematical model. For comparative purposes, the effects of norepinephrine were measured. The influence of Ni2+ on the cytosolic Ca2+ concentration ([Ca2+]c) in human hepatoma Huh7 cells and on liver glycogen catabolism, a biological response sensitive to cellular Ca2+, was also evaluated. The estimated transfer coefficients of 45Ca2+ transport indicated two mechanisms by which Ni2+ increases the [Ca2+]c in liver under steady-state conditions: (1) an increase in the net efflux of Ca2+ from intracellular Ca2+ stores due to a stimulus of Ca2+ efflux to the cytosolic space along with a diminution of Ca2+ re-entry into the cellular Ca2+ stores; (2) a decrease in Ca2+ efflux from the cytosolic space to vascular space, minimizing Ca2+ loss. Glycogen catabolism activated by Ni2+ was transient contrasting with the sustained activation induced by norepinephrine. Ni2+ caused a partial reduction in the norepinephrine-induced stimulation in the [Ca2+]c in Huh7 cells. Our data revealed that the kinetic parameters of Ca2+ transport modified by Ni2+ in intact liver are similar to those modified by norepinephrine in its first minutes of action, but the membrane receptors or Ca2+ transporters affected by Ni2+ seem to be distinct from those known to be modulated by norepinephrine.
Graphic abstract






Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Kumar S, Trivedi AV (2016) A review on role of nickel in the biological system. Int J Curr Microbiol Appl Sci 5:719–727. https://doi.org/10.20546/ijcmas.2016.503.084
Zambelli B, Ciurli S (2013) Nickel and human health. Met Ions Life Sci 13:321–357. https://doi.org/10.1007/978-94-007-7500-8_10
Tikare SN, Das GA, Dhundasi SA, Das KK (2008) Effect of antioxidants l-ascorbic acid and alpha-tocopherol supplementation in nickel exposed hyperglycemic rats. J Basic Clin Physiol Pharmacol 19:89–102. https://doi.org/10.1515/JBCPP.2008.19.2.89
Zelicoff JT, Thomas P (1998) Immunotoxicology of environmental and occupational metals. CRC Press, Boca Raton
Palermo FF, Risso WE, Simonato JD, Martinez CBR (2015) Bioaccumulation of nickel and its biochemical and genotoxic effects on juveniles of the neotropical fish Prochilodus lineatus. Ecotoxicol Environ Saf 116:19–28. https://doi.org/10.1016/j.ecoenv.2015.02.032
Sunderman FW, Aitio A, Morgan LG, Norseth T (1986) Biological monitoring of nickel. Toxicol Ind Health 2:17–78. https://doi.org/10.1177/074823378600200102
Vijver MG, Van Gestel CAM, Lanno RP et al (2004) Internal metal sequestration and its ecotoxicological relevance: a review. Environ Sci Technol 38:4705–4712. https://doi.org/10.1021/es040354g
Adjroud O (2013) The toxic effects of nickel chloride on liver, erythropoiesis, and development in Wistar albino preimplanted rats can be reversed with selenium pretreatment. Environ Toxicol 28:290–298. https://doi.org/10.1002/tox.20719
Buxton S, Garman E, Heim KE et al (2019) Concise review of nickel human health toxicology and ecotoxicology. Inorganics 7:89. https://doi.org/10.3390/inorganics7070089
Haber LT, Erdreicht L, Diamond GL et al (2000) Hazard identification and dose response of inhaled nickel-soluble salts. Regul Toxicol Pharmacol 31:210–230. https://doi.org/10.1006/rtph.2000.1377
Bai YN, Yang AM, Pu HQ et al (2014) Nickel-exposed workers in China: a cohort study. Biomed Environ Sci 27:208–211. https://doi.org/10.3967/bes2014.042
Coogan TP, Latta DM, Snow ET, Costa M (1989) Toxicity and carcinogenicity of nickel compounds. Crit Rev Toxicol 19:341–384. https://doi.org/10.3109/10408448909029327
Kasprzak KS, Sunderman FW, Salnikow K (2003) Nickel carcinogenesis. Mutat Res Fundam Mol Mech Mutagen 533:67–97. https://doi.org/10.1016/j.mrfmmm.2003.08.021
Novelli ELB, Rodrigues NL, Sforcin JM, Ribas BO (1997) Toxic effects of nickel exposure on heart and liver of rats. Toxic Subst Mech 16:251–258. https://doi.org/10.1080/107691897229612
Nieboer E (1975) The lanthanide ions as structural probes in biological and model systems. Struct Bond. https://doi.org/10.1007/bfb0116554
Evans CH (1990) Biochemistry of the Lanthanides. Plenum Press, New York
Adebanjo OA, Igietseme J, Huang CLH, Zaidi M (1998) The effect of extracellularly applied divalent cations on cytosolic Ca2+ in murine Leydig cells: evidence for a Ca2+-sensing receptor. J Physiol 513:399–410. https://doi.org/10.1111/j.1469-7793.1998.399bb.x
Cortijo J, Milara J, Mata M et al (2010) Nickel induces intracellular calcium mobilization and pathophysiological responses in human cultured airway epithelial cells. Chem Biol Interact 183:25–33. https://doi.org/10.1016/j.cbi.2009.09.011
McNulty TJ, Taylor CW (1999) Extracellular heavy-metal ions stimulate Ca2+ mobilization in hepatocytes. Biochem J 339:555–561. https://doi.org/10.1042/0264-6021:3390555
Handlogten ME, Shiraishi N, Awata H et al (2000) Extracellular Ca2+-sensing receptor is a promiscuous divalent cation sensor that responds to lead. Am J Physiol Ren Physiol 279:1083–1091. https://doi.org/10.1152/ajprenal.2000.279.6.f1083
Ward DT (2004) Calcium receptor-mediated intracellular signalling. Cell Calcium 35:217–228. https://doi.org/10.1016/j.ceca.2003.10.017
Brown EM, MacLeod RJ (2001) Extracellular calcium sensing and signalling. Physiol Rev 81:239–297. https://doi.org/10.1152/physrev.2001.81.1.239
Arias IM, Boyer JL, Fausto N et al (1994) The liver: biology and pathobiology. Raven Press, New York
Blazka ME, Shaikh ZA (1992) Cadmium and mercury accumulation in rat hepatocytes: interactions with other metal ions. Toxicol Appl Pharmacol 113:118–125. https://doi.org/10.1016/0041-008X(92)90015-K
Funakoshi T, Inoue T, Shimada H, Kojima S (1997) The mechanisms of nickel uptake by rat primary hepatocyte cultures: role of calcium channels. Toxicology 124:21–26. https://doi.org/10.1016/S0300-483X(97)00131-5
Boyer J (2002). In: Schiff ER, Sorrell MF, Maddrey WC (eds) Schiff’s diseases of the liver. Williams & Wilkins, Philadelphia
Dixon CJ, White PJ, Hall JF et al (2005) Regulation of human hepatocytes by P2Y receptors: control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J Pharmacol Exp Ther 313:1305–1313. https://doi.org/10.1124/jpet.104.082743
Karjalainen A, Bygrave FL (1995) Nickel: an agent for investigating the relation between hormone-induced Ca2+ influx and bile flow in the perfused rat liver. Cell Calcium 18:214–222. https://doi.org/10.1016/0143-4160(95)90066-7
Nathanson MH, Gautam A, Bruck R et al (1992) Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology 15:107–116. https://doi.org/10.1002/hep.1840150119
Hughes BP, Barritt GJ (1989) Inhibition of the liver cell receptor-activated Ca2+ inflow system by metal ion inhibitors of voltage-operated Ca2+ channels but not by other inhibitors of Ca2+ inflow. BBA - Mol Cell Res 1013:197–205. https://doi.org/10.1016/0167-4889(89)90135-3
Barritt GJ, Chen J, Rychkov GY (2008) Ca2+-permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology. Biochim Biophys Acta Mol Cell Res 1783:651–672. https://doi.org/10.1016/j.bbamcr.2008.01.016
Berridge MJ (2002) The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32:235–249. https://doi.org/10.1016/S0143416002001823
Zegers MMP, Hoekstra D (1998) Mechanisms and functional features of polarized membrane traffic in epithelial and hepatic cells. Biochem J 336:257–269. https://doi.org/10.1042/bj3360257
Utsunomiya KS, Scaliante LG, Bracht A, Ishii-Iwamoto EL (2013) Transport and distribution of 45Ca2+ in the perfused rat liver and the influence of adjuvant-induced arthritis. Biochim Biophys Acta Mol Basis Dis 1832:249–262. https://doi.org/10.1016/j.bbadis.2012.10.008
Ferraresi-Filho O, Ishii-Iwamoto EL, Bracht A (1997) Transport, metabolism and distribution space of octanoate in the perfused rat liver. Cell Biochem Funct 15:69–80. https://doi.org/10.1002/(SICI)1099-0844(19970601)15:2%3c69::AID-CBF721%3e3.0.CO;2-H
Goresky CA, Bach GC, Nadeau BE (1973) On the uptake of materials by the intact liver. The transport and net removal of galactose. J Clin Invest 52:991–1009. https://doi.org/10.1172/JCI107300
Schwab AJ, Bracht A, Scholz R (1979) Transport of d-Lactate in Perfused Rat Liver. Eur J Biochem 102:537–548. https://doi.org/10.1111/j.1432-1033.1979.tb04270.x
Fedatto-Júnior Z, Ishii-Iwamoto EL, Caparroz-Assef SM et al (2002) Glycogen levels and glycogen catabolism in livers from arthritic rats. Mol Cell Biochem 229:1–7. https://doi.org/10.1023/A:1017913124084
do Nascimento GS, Constantin RP, Gilglioni EH et al (2018) The acute effects of citrus flavanones on the metabolism of glycogen and monosaccharides in the isolated perfused rat liver. Toxicol Lett 291:158–172. https://doi.org/10.1016/j.toxlet.2018.04.001
Yamamoto NS, Ishii-Iwamoto EL, Bracht A (1992) Activation of glycogenolysis by methotrexate. Influence of calcium and inhibitors of hormone action. Biochem Pharmacol 44:761–767. https://doi.org/10.1016/0006-2952(92)90414-e
Nakabayashi H, Miyano K, Sato J et al (1982) Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res 42:3858–3863
Bracht A, Ishii-Iwamoto EL (2003) Métodos de Laboratório em Bioquímica. Manole, São Paulo
Clark LC (1956) Monitoring and control of blood O2 tension. Trans Am Soc Artif Intern Organs 2:41–49
Bracht A, Schwab AJ, Scholz R (1980) Untersuchung von flußgeschwindigkeiten in der isolierten perfundierten rattenleber durch pulsmarkierung mit radioaktiven substraten und mathematischer analyse der auswaschkinetiken. Hoppe Seylers Z Physiol Chem 361:357–378. https://doi.org/10.1515/bchm2.1980.361.1.357
Goresky CA, Ziegler WH, Bach GG, Wangel B (1970) Capillary exchange modeling. Circ Res 27:739–764. https://doi.org/10.1161/01.res.27.5.739
Rose CP, Goresky CA, Bach GG (1977) The capillary and sarcolemmal barriers in the heart. An exploration of labeled water permeability. Circ Res 41:515–533. https://doi.org/10.1161/01.RES.41.4.515
Björck A, Dahlquist G (1972) Numerische methoden. Oldenbourg Verlag, Munich
Wagon S (1999) Mathematica in action. Springer Science & Business Media, St. Paul
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450. https://doi.org/10.1016/s0021-9258(19)83641-4
Terracciano CMN, MacLeod KT (1996) Reloading of Ca2+-depleted sarcoplasmic reticulum during rest in guinea pig ventricular myocytes. Am J Physiol Hear Circ Physiol 271:H1814–H1822. https://doi.org/10.1152/ajpheart.1996.271.5.h1814
Bergmeyer HU (1974) Methods of enzymatic analysis. Academic Press, New York
Hansen CA, Yang L, Williamson JR (1991) Mechanisms of receptor-mediated Ca2+ signaling in rat hepatocytes. J Biol Chem 266:18573–18579. https://doi.org/10.1016/s0021-9258(18)55101-2
Putney JW Jr, Aub DL, Taylor CW, Merritt JE (1986) Formation and biological action of inositol 1,4,5-trisphosphate. Fed Proc 45:2634–2638
Reinhart PH, Taylor WM, Bygrave FL (1984) The action of α-adrenergic agonists on plasma-membrane calcium fluxes in perfused rat liver. Biochem J 220:43–50. https://doi.org/10.1042/bj2200043
Polato-Schmeisch A, Suzuki-Kemmelmeier F, Bracht A (2005) The Ca2+ mediated metabolic hemodynamic effects of vasopressin in the liver. Trends Cell Mol Biol 1:35–50
Withrington PG, Richardson PDI (1990). In: Zakim D, Boyer TD (eds) Hepatology. W.B. Saunders, Philadelphia
Shlatz L, Marinetti GV (1972) Calcium binding to the rat liver plasma membrane. Biochim Biophys Acta 290:70–83. https://doi.org/10.1016/0005-2736(72)90053-3
Kubrak OI, Rovenko BM, Husak VV et al (2012) Nickel induces hyperglycemia and glycogenolysis and affects the antioxidant system in liver and white muscle of goldfish Carassius auratus L. Ecotoxicol Environ Saf 80:231–237. https://doi.org/10.1016/j.ecoenv.2012.03.006
Gupta S, Ahmad N, Husain MM, Srivastava RC (2000) Involvement of nitric oxide in nickel-induced hyperglycemia in rats. Nitric Oxide Biol Chem 4:129–138. https://doi.org/10.1006/niox.2000.0278
Blackmore PF, Strickland WG, Bocckino SB, Exton JH (1986) Mechanism of hepatic glycogen synthase inactivation induced by Ca2+-mobilizing hormones. Studies using phospholipase C and phorbol myristate acetate. Biochem J 237:235–242. https://doi.org/10.1042/bj2370235
Kraus-Friedmann N, Feng L (1996) The role of intracellular Ca2+ in the regulation of gluconeogenesis. Metabolism 45:389–403. https://doi.org/10.1016/S0026-0495(96)90296-6
Barritt GJ, Litjens TL, Castro J et al (2009) Store-operated Ca2+ channels and microdomains of Ca2+ in liver cells. Clin Exp Pharmacol Physiol 36:77–83. https://doi.org/10.1111/j.1440-1681.2008.05095.x
Soboloff J, Rothberg BS, Madesh M, Gill DL (2012) STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol 13:549–565. https://doi.org/10.1038/nrm3414
Canaff L, Petit JL, Kisiel M et al (2001) Extracellular calcium-sensing receptor is expressed in rat hepatocytes. Coupling to intracellular calcium mobilization and stimulation of bile flow. J Biol Chem 276:4070–4079. https://doi.org/10.1074/jbc.M009317200
Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266:17067–17071. https://doi.org/10.1016/s0021-9258(19)47340-7
Zhang GH, Yamaguchi M, Kimura S et al (1990) Effects of heavy metal on rat liver microsomal Ca2+-ATPase and Ca2+-sequestering. Relation to SH groups. J Biol Chem 265:2184–2189. https://doi.org/10.1016/s0021-9258(19)39959-4
Carafoli E (1994) Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme 1. FASEB J 8:993–1002. https://doi.org/10.1096/fasebj.8.13.7926378
Prpic V, Green KC, Blackmore PF, Exton JH (1984) Vasopressin-, angiotensin II-, and α1-adrenergic-induced inhibition of Ca2+ transport by rat liver plasma membrane vesicles. J Biol Chem 259:1382–1385. https://doi.org/10.1016/s0021-9258(17)43414-4
Delgado-Coello B, Trejo R, Mas-Oliva J (2006) Is there a specific role for the plasma membrane Ca2+-ATPase in the hepatocyte? Mol Cell Biochem 285:1–15. https://doi.org/10.1007/s11010-005-9060-z
Jiang JL, Yu MK, Chen ZN, Chan HC (2001) cGMP-regulated store-operated calcium entry in human hepatoma cells. Cell Biol Int 25:993–995. https://doi.org/10.1006/cbir.2001.0751
Gregory RB, Rychkov G, Barritt GJ (2001) Does not involve inositol trisphosphate receptors. Society 290:285–290
Gao H, Wang F, Wang W, Makarewich CA, Zhang H, Kubo H, Berretta RM, Barr LA, Molkentin JD (2012) Ca2+ influx through L-type Ca2+ channels and transient receptor potential channels activate pathological hypertrophy signalling. J Mol Cell Cardiol 53:657–667. https://doi.org/10.1016/j.yjmcc.2012.08.005
Hobai IA, Hancox JC, Levi AJ (2000) Inhibition by nickel of the L-type Ca channel in guinea pig ventricular myocytes and effect of internal cAMP. Am J Physiol Hear Circ Physiol 279:692–701. https://doi.org/10.1152/ajpheart.2000.279.2.h692
Wani SA, Khan LA, Basir SF (2018) Role of calcium channels and endothelial factors in nickel induced aortic hypercontraction in Wistar rats. J Smooth Muscle Res 54:71–82. https://doi.org/10.1540/JSMR.54.71
Anke M, Angelow L, Glei M et al (1995) The biological importance of nickel in the food chain. Fresenius J Anal Chem 352:92–96. https://doi.org/10.1007/BF00322304
Grandjean P, Nielsen GD, Andersen O (1989). In: Maibach HI, Menné T (eds) Nickel and the skin: immunology and toxicology. CRC Press, Boca Raton
Varga I, Szebeni Á, Szoboszlai N, Kovács B (2005) Determination of trace elements in human liver biopsy samples by ICP-MS and TXRF: Hepatic steatosis and nickel accumulation. Anal Bioanal Chem 383:476–482. https://doi.org/10.1007/s00216-005-0010-0
Funding
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—308980/2017–4) and Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES/NUFFIC n°14/10). Karina Sayuri Utsunomiya holds fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Contributions
KSU: Investigation, validation, formal analysis, writing—original draft. LJdS: Investigation, validation. JI: Investigation, validation. RPC: Visualization, writing—review and editing. EHG: Visualization. JC: Writing—review and editing. AB: Methodology, software. RPJOE: Supervision, writing—review and editing. ELI-I: Supervision, project administration, funding acquisition, writing—original draft, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All experiments were conducted in strict adherence with the guidelines of the National Council for the Control of Animal Experimentation CONCEA (law no. 11.794 October 8, 2008) and The Ethics Committee on Animal Use (CEUA), of the State University of Maringa (Resolution n° 004/20160CEP), which follows the internationally accepted recommendations for the care and use of animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Utsunomiya, K.S., da Silva, L.J., Iwamoto, J. et al. Kinetic mechanisms by which nickel alters the calcium (Ca2+) transport in intact rat liver. J Biol Inorg Chem 26, 641–658 (2021). https://doi.org/10.1007/s00775-021-01883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01883-7