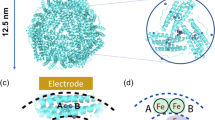
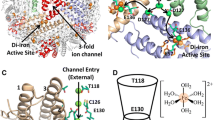
Abstract
In vitro, reductive mobilization of ferritin iron using suitable electron transfer mediators has emerged as a possible mechanism to mimic the iron release process, in vivo. Nature uses flavins as electron relay molecules for important biological oxidation and oxygenation reactions. Therefore, the current work utilizes three flavin analogues: riboflavin (RF), flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which differ in size and charge but have similar redox potentials, to relay electron from nicotinamide adenine dinucleotide (NADH) to ferritin mineral core. Of these, the smallest/neutral analogue, RF, released more iron (~ three fold) in comparison to the larger and negatively charged FMN and FAD. Although iron mobilization got marred during the initial stages under aerobic conditions, but increased with a greater slope at the later stages of the reaction kinetics, which gets inhibited by superoxide dismutase, consistent with the generation of O2∙− in situ. The initial step, i.e., interaction of flavins with NADH played critical role in the iron release process. Overall, the flavin-mediated reductive iron mobilization from ferritins occurred via two competitive pathways, involving the reduced form of flavins either alone (anaerobic condition) or in combination with O2∙− intermediate (aerobic condition). Moreover, faster iron release was observed for ferritins from Mycobacterium tuberculosis than from bullfrog, indicating the importance of protein nanocage and the advantages they provide to the respective organisms. Therefore, these structure–reactivity studies of flavins with NADH/O2 holds significance in ferritin iron release, bioenergetics, O2-based cellular toxicity and may be potentially exploited in the treatment of methemoglobinemia.
Graphic abstract
Smaller sized/neutral flavin analogue, riboflavin (RF) exhibits faster reactivity towards both NADH and O2 generating more amount of O2∙− and releases higher amount of iron from different ferritins, compared to its larger sized/negatively charged derivatives such as FMN and FAD.










Similar content being viewed by others
Abbreviations
- ET:
-
Electron transfer
- LZ:
-
Lumazine
- RF:
-
Riboflavin
- FMN:
-
Flavin mononucleotide
- FAD:
-
Flavin adenine dinucleotide
- Fox :
-
Ferroxidase center
- Mtb :
-
Mycobacterium tuberculosis
- BfrA:
-
Bacterioferritin A
- BfrB:
-
Bacterioferritin B
- Frog M:
-
Bullfrog ferritin with M subunits
- PAGE:
-
Poly-acrylamide gel electrophoresis
- E 1/2 :
-
Midpoint potential
- CV:
-
Cyclic voltammetry
- SWV:
-
Square wave voltammetry
- DPV:
-
Differential pulsed voltammetry
- DMSO:
-
Dimethyl sulfoxide
- MOPS:
-
3-(N-Morpholino) propane sulfonic acid
- NADH:
-
β-Nicotinamide adenine dinucleotide
- ROS:
-
Reactive oxygen species
- Flox :
-
Oxidized flavin quinone
- Flsq :
-
1 e− reduced flavin semi-quinone
- Flred :
-
2 e− reduced flavin hydroquinone
- Fz:
-
Ferrozine
- Tyr:
-
Tyrosine
- Trp:
-
Tryptophan
- pI:
-
Isoelectric point
- PDB:
-
Protein data bank
- MM-GBSA:
-
Molecular Mechanics energies combined with the Generalized Born and Surface Area continuum solvation
- MD:
-
Molecular docking
References
Theil EC, Tosha T, Behera RK (2016) Acc Chem Res 49:784–791
Sheftel AD, Mason AB, Ponka P (2012) Biochimica et Biophysica Acta (BBA) General Subjects 1820:161–187
Crichton R (2009) Iron metabolism. Wiley, New York, pp 17–58
Theil EC, Behera RK, Tosha T (2013) Coord Chem Rev 257:579–586
Bou-Abdallah F (2010) Biochem Biophys Acta 1800:719–731
Mohanty A, Subhadarshanee B, Barman P, Mahapatra C, Aishwarya B, Behera RK (2019) Inorg Chem 58:4741–4752
Parida A, Mohanty A, Kansara BT, Behera RK (2020) Inorg Chem 59:629–641
Theil EC, Behera RK (2013) Coordination chemistry in protein cages. Wiley, New York, pp 3–24
Honarmand Ebrahimi K, Hagedoorn PL, Hagen WR (2015) Chem Rev 115:295–326
Tosha T, Ng H-L, Bhattasali O, Alber T, Theil EC (2010) J Am Chem Soc 132:14562–14569
Bernacchioni C, Ghini V, Theil EC, Turano P (2016) RSC Adv 6:21219–21227
Behera RK, Torres R, Tosha T, Bradley JM, Goulding CW, Theil EC (2015) J Biol Inorg Chem 20:957–969
Behera RK, Theil EC (2014) Proc Natl Acad Sci USA 111:7925–7930
Maity B, Fujita K, Ueno T (2015) Curr Opin Chem Biol 25:88–97
Tosha T, Behera RK, Ng HL, Bhattasali O, Alber T, Theil EC (2012) J Biol Chem 287:13016–13025
Treffry A, Bauminger ER, Hechel D, Hodson NW, Nowik I, Yewdall SJ, Harrison PM (1993) Biochem J 296(Pt 3):721–728
Bertini I, Lalli D, Mangani S, Pozzi C, Rosa C, Theil EC, Turano P (2012) J Am Chem Soc 134:6169–6176
Pozzi C, Pisa F, Lalli D, Rosa C, Theil E, Turano P, Mangani S (2015) Acta Crystallogr Sect D Biol Crystallogr 71:941–953
Bernacchioni C, Pozzi C, Di Pisa F, Mangani S, Turano P (2016) Chem Eur J 22:16213–16219
Koochana PK, Mohanty A, Das S, Subhadarshanee B, Satpati S, Dixit A, Sabat SC, Behera RK (2018) Biochem Biophys Acta 1862:1190–1198
Rivera M (2017) Acc Chem Res 50:331–340
Mehlenbacher M, Poli M, Arosio P, Santambrogio P, Levi S, Chasteen ND, Bou-Abdallah F (2017) Biochemistry 56:3900–3912
Bradley JM, Moore GR, Le Brun NE (2014) J Biol Inorg Chem 19:775–785
Chasteen ND, Harrison PM (1999) J Struct Biol 126:182–194
Kwak Y, Schwartz JK, Haldar S, Behera RK, Tosha T, Theil EC, Solomon EI (2014) Biochemistry 53:473–482
Bradley JM, Svistunenko DA, Pullin J, Hill N, Stuart RK, Palenik B, Wilson MT, Hemmings AM, Moore GR, Le Brun NE (2019) Proc Natl Acad Sci 116:2058
Bradley JM, Le Brun NE, Moore GR (2016) J Biol Inorg Chem 21:13–28
Ceci P, Di Cecca G, Falconi M, Oteri F, Zamparelli C, Chiancone E (2011) J Biol Inorg Chem 16:869–880
Gálvez N, Fernández B, Sánchez P, Cuesta R, Ceolín M, Clemente-León M, Trasobares S, López-Haro M, Calvino JJ, Stéphan O, Domínguez-Vera JM (2008) J Am Chem Soc 130:8062–8068
Pandey R, Rodriguez GM (2014) Mol Microbiol 91:98–109
Theil EC (2013) Inorg Chem 52:12223–12233
Khare G, Nangpal P, Tyagi AK (2017) PLoS ONE 12:e0169545
Maity B, Hishikawa Y, Lu D, Ueno T (2019) Polyhedron 172:104–111
Koochana PK, Mohanty A, Subhadarshanee B, Satpati S, Naskar R, Dixit A, Behera RK (2019) Dalton Trans 48:3314–3326
Melman G, Bou-Abdallah F, Vane E, Maura P, Arosio P, Melman A (2013) Biochimica et Biophysica Acta BBA General Subj 1830:4669–4674
Badu-Boateng C, Naftalin RJ (2019) Free Radical Biol Med 133:75–87
Kidane TZ, Sauble E, Linder MC (2006) Am J Physiol Cell Physiol 291:C445-455
Sala D, Ciambellotti S, Giachetti A, Turano P, Rosato A (2017) J Chem Inf Model 57:2112–2118
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014) Nature 509:105–109
Gryzik M, Srivastava A, Longhi G, Bertuzzi M, Gianoncelli A, Carmona F, Poli M, Arosio P (2017) Biochem Biophys Acta 1861:2710–2716
Badu-Boateng C, Naftalin RJ (2018). Free Radical Biol Med. https://doi.org/10.1016/j.freeradbiomed.2018.09.041
Jones T, Spencer R, Walsh C (1978) Biochemistry 17:4011–4017
Yao H, Wang Y, Lovell S, Kumar R, Ruvinsky AM, Battaile KP, Vakser IA, Rivera M (2012) J Am Chem Soc 134:13470–13481
Eshelman K, Yao H, Punchi Hewage AND, Deay JJ, Chandler JR, Rivera M (2017) Met Integr Biometal Sci 9:646–659
De Domenico I, Ward DM, Kaplan J (2009) Blood 114:4546–4551
Watt RK, Hilton RJ, Graff DM (2010) Biochimica et Biophysica Acta (BBA) General Subj 1800:745–759
Bou-Abdallah F, McNally J, Liu XX, Melman A (2011) Chem Commun 47:731–733
Soldano A, Yao H, Punchi Hewage AND, Meraz K, Annor-Gyamfi JK, Bunce RA, Battaile KP, Lovell S, Rivera M (2020). ACS Infectious Diseases. https://doi.org/10.1021/acsinfecdis.0c00669
Punchi Hewage AND, Yao H, Nammalwar B, Gnanasekaran KK, Lovell S, Bunce RA, Eshelman K, Phaniraj SM, Lee MM, Peterson BR, Battaile KP, Reitz AB, Rivera M (2019) J Am Chem Soc 141:8171–8184
Massey V (1994) J Biol Chem 269:22459–22462
Buckel W, Thauer RK (2018) Chem Rev 118:3862–3886
Romero E, Gómez Castellanos JR, Gadda G, Fraaije MW, Mattevi A (2018) Chem Rev 118:1742–1769
Weber S, Schleicher E (2014) Flavins and flavoproteins: methods and protocols. Springer Science, New York
Banerjee R, Becker DF, Dickman MB, Gladyshev VN, Ragsdale SW (2007) Redox biochemistry. Wiley, New York
Subramanian V, Evans DG (2012) J Phys Chem B 116:9287–9302
Ulvik RJ, Romslo I, Roland F, Crichton RR (1981) Biochimica et Biophysica Acta (BBA)- General Subj 677:50–56
Satoh J, Kimata S, Nakamoto S, Ishii T, Tanaka E, Yumoto S, Takeda K, Yoshimura E, Kanesaki Y, Ishige T, Tanaka K, Abe A, Kawasaki S, Niimura Y (2019) J General Appl Microbiol 65:308–315
Takaishi K, Kitahata H (2019) J Med Invest 66:230–232
Subhadarshanee B, Mohanty A, Jagdev MK, Vasudevan D, Behera RK (2017) Biochem Biophys Acta 1865:1267–1273
Khare G, Gupta V, Nangpal P, Gupta RK, Sauter NK, Tyagi AK (2011) PLoS ONE 6:e18570
Gupta V, Gupta RK, Khare G, Salunke DM, Tyagi AK (2009) PLoS ONE 4:e8028
Tosha T, Hasan MR, Theil EC (2008) Proc Natl Acad Sci 105:18182
Behera RK, Nakajima H, Rajbongshi J, Watanabe Y, Mazumdar S (2013) Biochemistry 52:1373–1384
Donlin MJ, Frey RF, Putnam C, Proctor J, Bashkin JK (1998) J Chem Educ 75:437
Yasmin S, Andrews SC, Moore GR, Le Brun NE (2011) J Biol Chem 286:3473–3483
Roy B, Krishnan SP, Chandrasekaran N, Mukherjee A (2019) In: Verma SK, Das AK (eds) Toxic effects of engineered nanoparticles (metal/metal oxides) on plants using Allium cepa as a model system, Comprehensive analytical chemistry. Elsevier, Amsterdam, pp 125–143
Diculescu VC, Militaru A, Shah A, Qureshi R, Tugulea L, Brett AMO (2010) J Electroanal Chem 647:1–7
Tatur J, Hagen WR, Heering HA (2009). Dalton Trans. https://doi.org/10.1039/b819775j:2837-2842
Jameson GN, Jameson RF, Linert W (2004) Org Biomol Chem 2:2346–2351
70F. Bou-Abdallah, J. J. Paliakkara, G. Melman and A. Melman (2018) Pharmaceuticals 11
Winkler JR, Gray HB (2014) J Am Chem Soc 136:2930–2939
Giese B, Wang M, Gao J, Stoltz M, Müller P, Graber M (2009) J Org Chem 74:3621–3625
Place TL, Domann FE, Case AJ (2017) Free Radical Biol Med 113:311–322
Kuriyan J, Konforti B, Wemmer D (2013) The molecules of life: physical and chemical principles. Garland Science, New York
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller A-F, Teixeira M, Valentine JS (2014) Chem Rev 114:3854–3918
Johnson LE, Wilkinson T, Arosio P, Melman A, Bou-Abdallah F (2017) Biochimica et Biophysica Acta (BBA) - General Subj 1861:3257–3262
Douglas T, Ripoll DR (1998) Protein Sci 7:1083–1091
Chandramouli B, Bernacchioni C, Di Maio D, Turano P, Brancato G (2016) J Biol Chem 291:25617–25628
Bellapadrona G, Stefanini S, Zamparelli C, Theil EC, Chiancone E (2009) J Biol Chem 284:19101–19109
Muhoberac BB, Vidal R (2019) Front Neurosci 13:1195
Kettisen K, Bülow L, Sakai H (2015) Bioconjug Chem 26:746–754
Acknowledgements
This work was supported by Science and Engineering Research Board (SERB), India (EMR/2016/003894) to R.K.B. and P.K.K., and Department of Biotechnology (DBT), India (BT/PR22042/NNT/28/1247/2017) to R.K.B. and A.P. Authors are thankful to Dr. Elizabeth C. Theil (C.H.O.R.I., USA), Dr. Anil K. Tyagi and Dr. Garima Khare (University of Delhi South Campus, India) and to Dr. Anadi C. Dash (NISER, India) and Dr. Ruma Banerjee (University of Michigan, USA) for their generous support in providing the ferritin clones and for their critical suggestions, respectively. The authors are also thankful to Ms. Sunita Dhaka and Mr. Jayanta Kumar Murmu for their experimental assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Dedicated to Prof. Elizabeth C. Theil (Professor—Emeritus, CHORI and NCSU, USA).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic Supplementary Material
Details of isoelectric point and charge at neutral pH for frog M and Mycobacterial ferritins, electrochemical (cyclic, square wave and differential pulse voltammograms) analysis of flavin mediators, kinetics and initial rates of iron release by flavin mediators from different ferritins, kinetics of NADH oxidation and dissolved O2 consumption at different concentration of flavin mediators, kinetics of iron release by discontinuous ferrozine assay, effect of SOD and kinetics of formation of formazan at different time intervals, kinetics of iron release by flavins under anaerobic conditions and in the presence of SOD and molecular docking results for NADH-flavins/flavins-ferritins interaction. (PDF 2200 KB)
Rights and permissions
About this article
Cite this article
Koochana, P.K., Mohanty, A., Parida, A. et al. Flavin-mediated reductive iron mobilization from frog M and Mycobacterial ferritins: impact of their size, charge and reactivities with NADH/O2 . J Biol Inorg Chem 26, 265–281 (2021). https://doi.org/10.1007/s00775-021-01850-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01850-2