Abstract
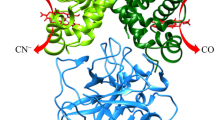
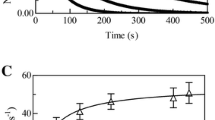
Haptoglobin (Hp) binds human hemoglobin (Hb), contributing to prevent extra-erythrocytic Hb-induced damage. Hp forms preferentially complexes with αβ dimers, displaying heme-based reactivity. Here, kinetics and thermodynamics of fluoride and azide binding to ferric human Hb (Hb(III)) complexed with the human Hp phenotypes 1-1 and 2-2 (Hp1-1:Hb(III) and Hp2-2:Hb(III), respectively) are reported (pH 7.0 and 20.0 °C). Fluoride binds to Hp1-1:Hb(III) and Hp2-2:Hb(III) with a one-step kinetic and equilibrium behavior. In contrast, kinetics of azide binding to and dissociation from Hp1-1:Hb(III)(–N3−) and Hp2-2:Hb(III)(–N3−) follow a two-step process. However, azide binding to Hp1-1:Hb(III) and Hp2-2:Hb(III) is characterized by a simple equilibrium, reflecting the compensation of kinetic parameters. The fast and the slow step of azide binding to Hp1-1:Hb(III) and Hp2-2:Hb(III) should reflect azide binding to the ferric β and α chains, respectively, as also proposed for the similar behavior observed in Hb(III). Present results highlight the ligand-dependent kinetic inequivalence of Hb subunits in the ferric form, reflecting structural differences between the two subunits in the interaction with some ferric ligands.
Graphical abstract





Similar content being viewed by others
Abbreviations
- CCP domain:
-
Complement control protein domain
- Hb:
-
Human hemoglobin
- Hb(III):
-
Ferric Hb
- Hp:
-
Human haptoglobin
- Hp1-1:
-
Phenotype 1-1 of Hp
- Hp2-2:
-
Phenotype 2-2 of Hp
- Hp1-1:Hb(III):
-
Ferric Hp1-1:Hb complex
- Hp2-2:Hb(III):
-
Ferric Hp 2-2:Hb complex
- SP-like domain:
-
Serine protease-like domain
References
Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M (1968) Blood 32:811–815
Bunn HF, Forget BG (1986) Hemoglobin: molecular, genetic and clinical aspects. Saunders, Philadelphia
Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M (2005) IUBMB Life 57:749–759
Alayash AI, Andersen CB, Moestrup SK, Bülow L (2013) Trends Biotechnol 31:2–3
Alayash AI (2004) Nat Rev Drug Discov 3:152–159
Schaer DJ, Vinchi F, Ingoglia G, Tolosano E, Buehler PW (2014) Front Physiol 5:415
MacKellar M, Vigerust DJ (2016) Clin Diabetes 34:148–157
Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Nature 409:198–201
Buehler PW, Abraham B, Vallelian F, Linnemayr C, Pereira CP, Cipollo JF, Jia Y, Mikolajczyk M, Boretti FS, Schoedon G, Alayash AI, Schaer DJ (2009) Blood 113:2578–2586
Kaempfer T, Duerst E, Gehrig P, Roschitzki B, Rutishauser D, Grossmann J, Schoedon G, Vallelian F, Schaer DJ (2011) J Proteome Res 10:2397–2408
Andersen CBF, Stødkilde K, Sæderup KL, Kuhlee A, Raunser S, Graversen JH, Moestrup SK (2017) Antioxid Redox Signal 26:814–831
Polticelli F, Bocedi A, Minervini G, Ascenzi P (2008) FEBS J 275:5648–5656
Andersen CB, Torvund-Jensen M, Nielsen MJ, de Oliveira CL, Hersleth HP, Andersen NH, Pedersen JS, Andersen GR, Moestrup SK (2012) Nature 489:456–459
Stødkilde K, Torvund-Jensen M, Moestrup SK, Andersen CB (2014) Nat Commun 5:5487
Kurosky A, Barnett DR, Lee TH, Touchstone B, Hay RE, Arnott MS, Bowman BH, Fitch WM (1980) Proc Natl Acad Sci USA 77:3388–3392
Wejman JC, Hovsepian D, Wall JS, Hainfeld JF, Greer J (1984) J Mol Biol 174:319–341
Wejman JC, Hovsepian D, Wall JS, Hainfeld JF, Greer J (1984) J Mol Biol 174:343–368
Nagel RL, Gibson QH (1971) J Biol Chem 246:69–73
Nagel RL, Wittenberg JB, Ranney HM (1965) Biochim Biophys Acta 100:286–289
Nagel RL, Gibson QH (1966) J Mol Biol 22:249–255
Brunori M, Alfsen A, Saggese U, Antonini E, Wyman J (1968) J Biol Chem 243:2950–2954
Gibson QH, Parkhurst LJ, Geraci G (1969) J Biol Chem 244:4668–4676
Alfsen A, Chiancone E, Antonini E, Waks M, Wyman J (1970) Biochim Biophys Acta 207:395–403
Antonini E, Brunori M (1971) Hemoglobin and myoglobin in their reactions with ligands. North Holland Publishing Co, Amsterdam
Chiancone E, Antonini E, Brunori M, Alfsen A, Lavialle F (1973) Biochem J 133:205–207
Perutz MF (1979) Annu Rev Biochem 48:327–386
Azarov I, He X, Jeffers A, Basu S, Ucer B, Hantgan RR, Levy A, Kim-Shapiro DB (2008) Nitric Oxide 18:296–302
Ascenzi P, Tundo GR, Coletta M (2018) J Inorg Biochem 187:116–122
Coletta M, Angeletti M, De Sanctis G, Cerroni L, Giardina B, Amiconi G, Ascenzi P (1996) Eur J Biochem 235:49–53
Ackers GK, Doyle ML, Myers D, Daugherty MA (1992) Science 255:54–63
White SL (1975) J Biol Chem 250:1263–1268
Ascenzi P, De Simone G, Polticelli F, Gioia M, Coletta M (2018) J Biol Inorg Chem 23:437–445
Ascenzi P, Coletta M (2018) J Phys Chem B 122:11100–11107
Herold S, Shivashankar K (2003) Biochemistry 42:14036–14046
Klapper MH, Uchida H (1971) J Biol Chem 246:6849–6854
Deatherage JF, Loe RS, Moffat K (1976) J Mol Biol 104:723–728
Deatherage JF, Obendorf SK, Moffat K (1979) J Mol Biol 134:419–429
Banerjee S, Jia Y, Siburt CJ, Abraham B, Wood F, Bonaventura C, Henkens R, Crumbliss AL, Alayash AI (2012) Free Radic Biol Med 53:1317–1326
Anusiem AC, Beetlestone JG, Irvine DH (1968) J Chem Soc A 960–969
Bailey JE, Beetlestone JG, Irvine DH (1968) J Chem Soc A 2778–2783
Acknowledgements
The Grant of Dipartimenti di Eccellenza, MIUR (Legge 232/2016, Articolo 1, Comma 314-337) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ascenzi, P., di Masi, A., De Simone, G. et al. Fluoride and azide binding to ferric human hemoglobin:haptoglobin complexes highlights the ligand-dependent inequivalence of the α and β hemoglobin chains. J Biol Inorg Chem 24, 247–255 (2019). https://doi.org/10.1007/s00775-019-01642-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-019-01642-9